Human Milk Oligosaccharides (HMOs)
Complex sugars naturally found in breast milk that shape the infant gut microbiome and are now available as supplements for gut and immune health.
Food Sources
Naturally found in these foods:
Key Benefits
- Establishes healthy infant microbiome
- Selective Bifidobacterium feeding
- Pathogen defense
- Immune system development
- Gut barrier support
Bacteria This Prebiotic Feeds
This prebiotic selectively nourishes these beneficial microorganisms:
Overview
Human milk oligosaccharides (HMOs) are a diverse group of complex sugars that represent the third most abundant solid component in human breast milk, after lactose and lipids[1]. Over 200 distinct HMO structures have been identified, collectively present at concentrations of 5-20 g/L in mature milk and up to 23 g/L in colostrum. Unlike digestible nutrients, HMOs pass through the infant's digestive system largely intact, serving as prebiotics that selectively nourish beneficial bacteria—particularly Bifidobacterium species adapted to utilize these complex sugars.
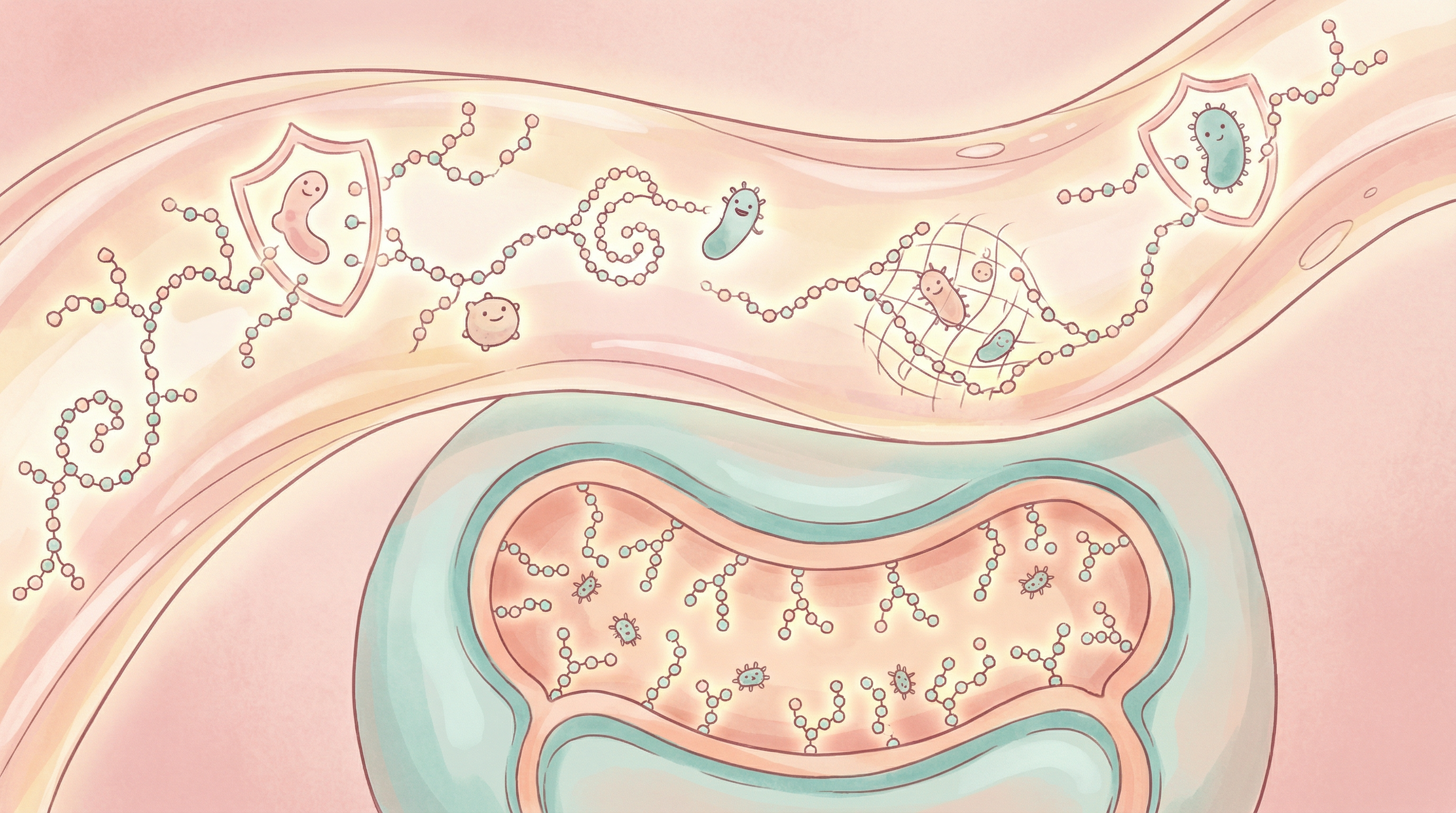
Structure and Diversity
HMOs share a common structural framework[1]:
Core Structure
- Lactose core: All HMOs are built on a lactose foundation
- Extended by: N-acetylglucosamine, galactose, fucose, and sialic acid
- Chain length: Ranges from 3 to over 32 sugar units
- Fucosylation: ~70% of HMOs contain fucose residues
- Sialylation: ~30% contain sialic acid
Major HMOs
The most abundant and studied HMOs include:
| HMO | Type | Prevalence |
|---|---|---|
| 2'-Fucosyllactose (2'-FL) | Neutral, fucosylated | Most abundant (~30%) |
| Lacto-N-tetraose (LNT) | Neutral, non-fucosylated | Major HMO |
| Lacto-N-neotetraose (LNnT) | Neutral, non-fucosylated | Major HMO |
| 3'-Sialyllactose (3'-SL) | Acidic, sialylated | Common |
| 6'-Sialyllactose (6'-SL) | Acidic, sialylated | Common |
Secretor Status
HMO composition varies based on maternal genetics[1]:
- Secretors (~80%): Produce α1,2-fucosylated HMOs (including 2'-FL)
- Non-secretors (~20%): Lack FUT2 gene expression, different HMO profile
- This affects which Bifidobacterium strains thrive in infants
Mechanism of Action
Selective Prebiotic Effect
HMOs serve as the primary food source for specific beneficial bacteria[2]:
- Species-specific utilization: Bifidobacterium longum subsp. infantis possesses complete genetic machinery to consume all HMO types
- Enzymatic degradation: Infant-adapted bifidobacteria have evolved unique glycosyl hydrolases for HMO breakdown
- Competitive exclusion: Rapid HMO consumption by bifidobacteria outcompetes pathogens for resources
- SCFA production: Fermentation produces acetate and lactate, lowering gut pH
B. infantis has been identified as the "champion colonizer" of the infant gut due to its specialized HMO utilization capabilities[3]. This subspecies can import intact HMOs and digest them intracellularly, a strategy that maximizes energy capture and minimizes nutrient sharing with competitors.
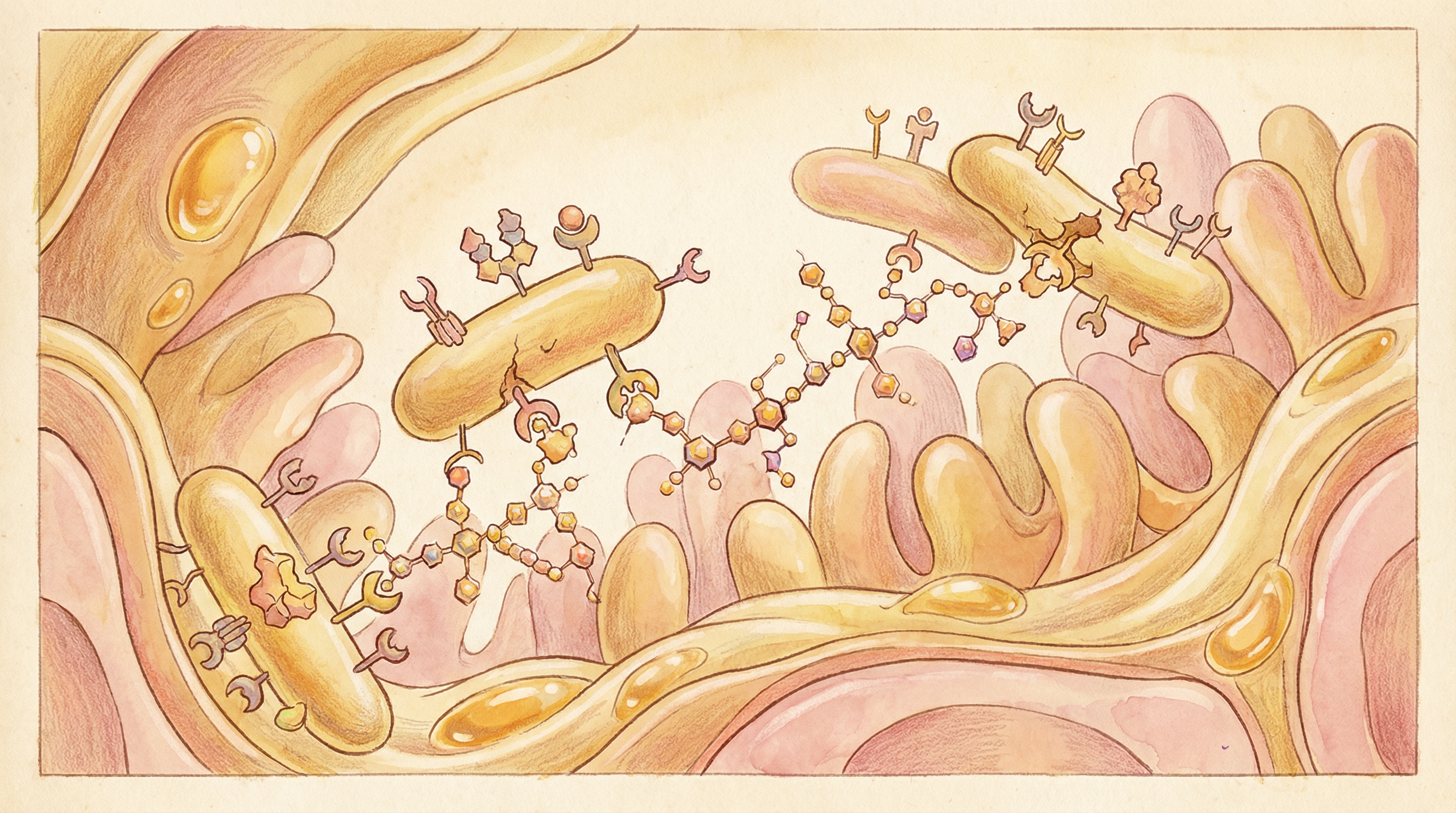
Pathogen Defense
HMOs protect against infections through multiple mechanisms[4]:
- Receptor decoys: HMOs structurally mimic glycan receptors on intestinal cells
- Pathogen binding: Block attachment of viruses, bacteria, and parasites
- Direct antimicrobial: Some HMOs have direct bacteriostatic effects
- Biofilm prevention: Inhibit pathogen biofilm formation
Specific examples include:
- 2'-FL blocks Campylobacter jejuni adhesion
- Sialylated HMOs reduce influenza virus binding
- Multiple HMOs inhibit Entamoeba histolytica
Immune Modulation
Beyond prebiotic effects, HMOs directly influence immune development[6]:
- Modulate dendritic cell and T-cell responses
- Influence intestinal epithelial cell signaling
- Support gut barrier development
- May reduce allergy and autoimmune disease risk
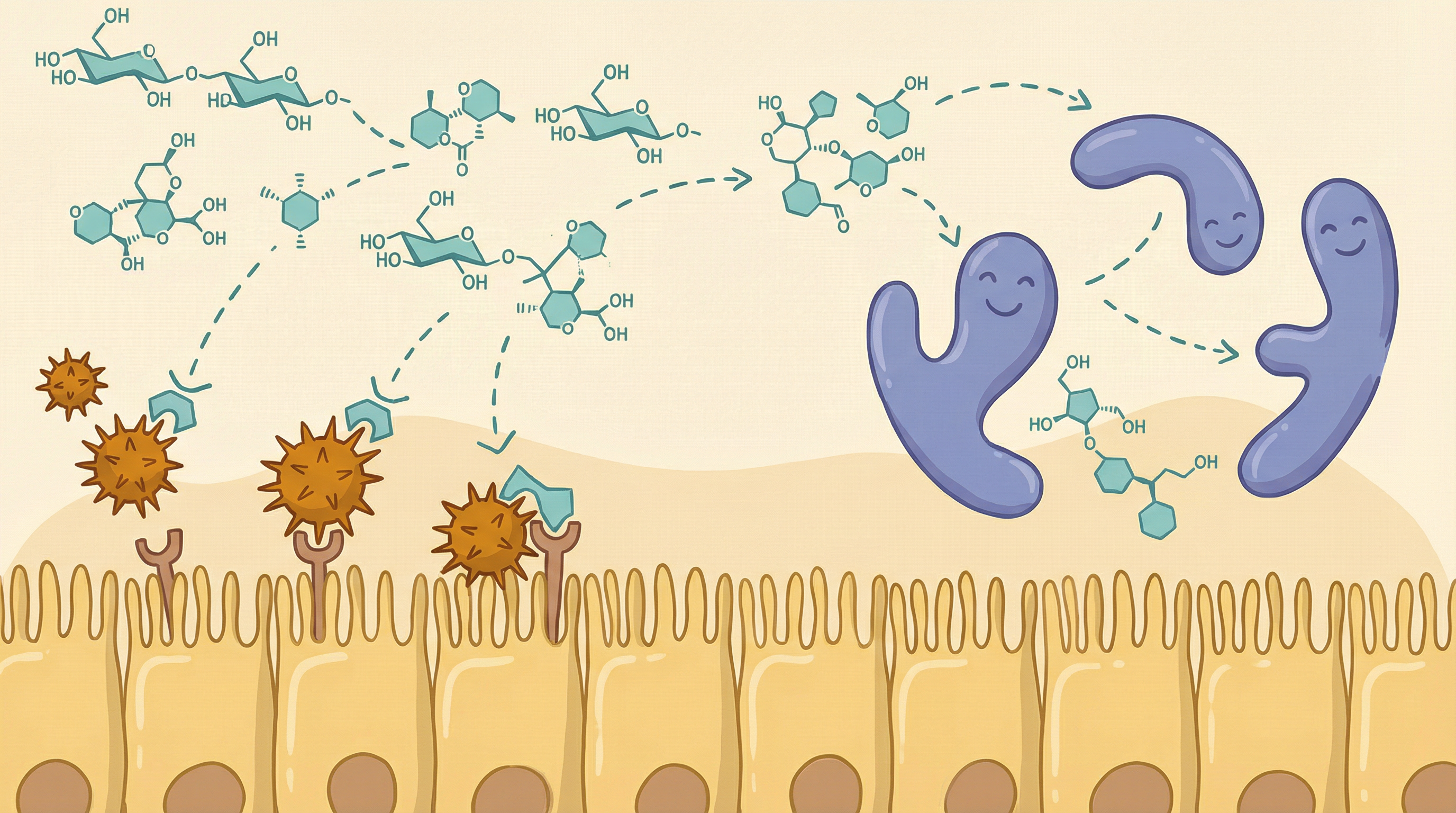
Effects on Gut Microbiome
Infant Microbiome Development
HMOs play a central role in establishing the infant gut microbiome[2]:
- Bifidobacterium dominance: Breastfed infants have microbiomes dominated by bifidobacteria (60-90%)
- Species selection: B. infantis, B. bifidum, and B. breve are selectively enriched
- Formula-fed contrast: Without HMOs, formula-fed infants develop more adult-like, diverse microbiomes
Adult Effects
Recent studies show HMOs also benefit adults[5]:
- Supplementation with 2'-FL and LNnT is well-tolerated
- Shifts intestinal microbiota toward increased Bifidobacterium
- Actinobacteria (including Bifidobacterium) increased 5-fold with 10g 2'-FL
- Effects are dose-dependent
Clinical Evidence
Infant Health Outcomes
Breastfeeding and HMO exposure are associated with[1]:
- Reduced incidence of diarrheal diseases
- Lower respiratory infection rates
- Decreased risk of necrotizing enterocolitis (NEC) in preterm infants
- Potential protection against allergies and asthma
Adult Supplementation
A landmark study on adult HMO supplementation demonstrated[5]:
- Good tolerability: Up to 20g/day of 2'-FL well-tolerated
- Microbiome shifts: Significant increases in Bifidobacterium
- Dose-response: Higher doses produced greater bifidogenic effects
- No adverse effects: Safety comparable to placebo
Immune Benefits
HMOs have been shown to:
- Reduce inflammation in gut epithelium
- Enhance vaccine responses
- Support barrier function
- Modulate allergic responses
Commercial Availability
HMOs in Infant Formula
Several HMOs are now added to infant formulas[6]:
- 2'-Fucosyllactose (2'-FL): First HMO approved for formula use
- Lacto-N-neotetraose (LNnT): Available in some formulas
- Produced through fermentation or enzymatic synthesis
- Levels typically 0.2-1.0 g/L (lower than breast milk)
Adult Supplements
HMOs are increasingly available as supplements:
- Pure 2'-FL products
- HMO blends
- Combination with other prebiotics
- Targeted gut health formulations
Dosage Considerations
Infants (via breast milk or formula)
- Breast milk provides 5-20 g/L HMOs
- Formula supplementation: 0.2-1.0 g/L
- Natural variation based on lactation stage
Adults
Based on clinical evidence[5]:
- Effective range: 2-20g daily
- Typical supplementation: 1-5g daily
- Tolerability: Excellent up to 20g/day
- Duration: Effects seen within 2-3 weeks
Safety Profile
HMOs have an excellent safety profile:
- Consumed by humans throughout evolutionary history
- No adverse effects in clinical trials
- Well-tolerated at high doses
- FDA GRAS status for 2'-FL
- Approved for infant formula use globally
Future Directions
Research is expanding on:
- Larger panels of HMOs for supplementation
- Precision matching of HMOs to individual microbiomes
- Synbiotic combinations with B. infantis
- Applications in immune disorders and allergies
- Adult health optimization
Summary
Human milk oligosaccharides represent nature's original prebiotics, evolved over millions of years to shape the infant gut microbiome and support healthy development. Their highly selective promotion of beneficial Bifidobacterium species, combined with direct pathogen defense and immune modulation, makes them uniquely valuable for gut health. With commercial availability expanding beyond infant formula to adult supplements, HMOs offer evidence-based prebiotic benefits across the lifespan, supported by their exceptional safety profile and potent bifidogenic effects.
Dosage Guidelines
Recommended Dosage
1-5g daily (supplements)
Start with a lower dose and gradually increase to minimize digestive discomfort. Consult a healthcare provider for personalized recommendations.
References
- Bode L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology. 2012;22(9):1147-1162. doi:10.1093/glycob/cws074
- Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends in Microbiology. 2010;18(7):298-307. doi:10.1016/j.tim.2010.03.008
- Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatric Research. 2015;77(1-2):229-235. doi:10.1038/pr.2014.156
- Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annual Review of Nutrition. 2005;25:37-58. doi:10.1146/annurev.nutr.25.050304.092553
- Elison E, Vigsnaes LK, Rinber Krogsgaard L, et al.. Oral supplementation of healthy adults with 2′-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. British Journal of Nutrition. 2016;116(8):1356-1368. doi:10.1017/S0007114516003354
- Walsh C, Lane JA, van Sinderen D, Hickey RM. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. Journal of Functional Foods. 2020;72:104074. doi:10.1016/j.jff.2020.104074
