Bordetella pertussis
Key Characteristics
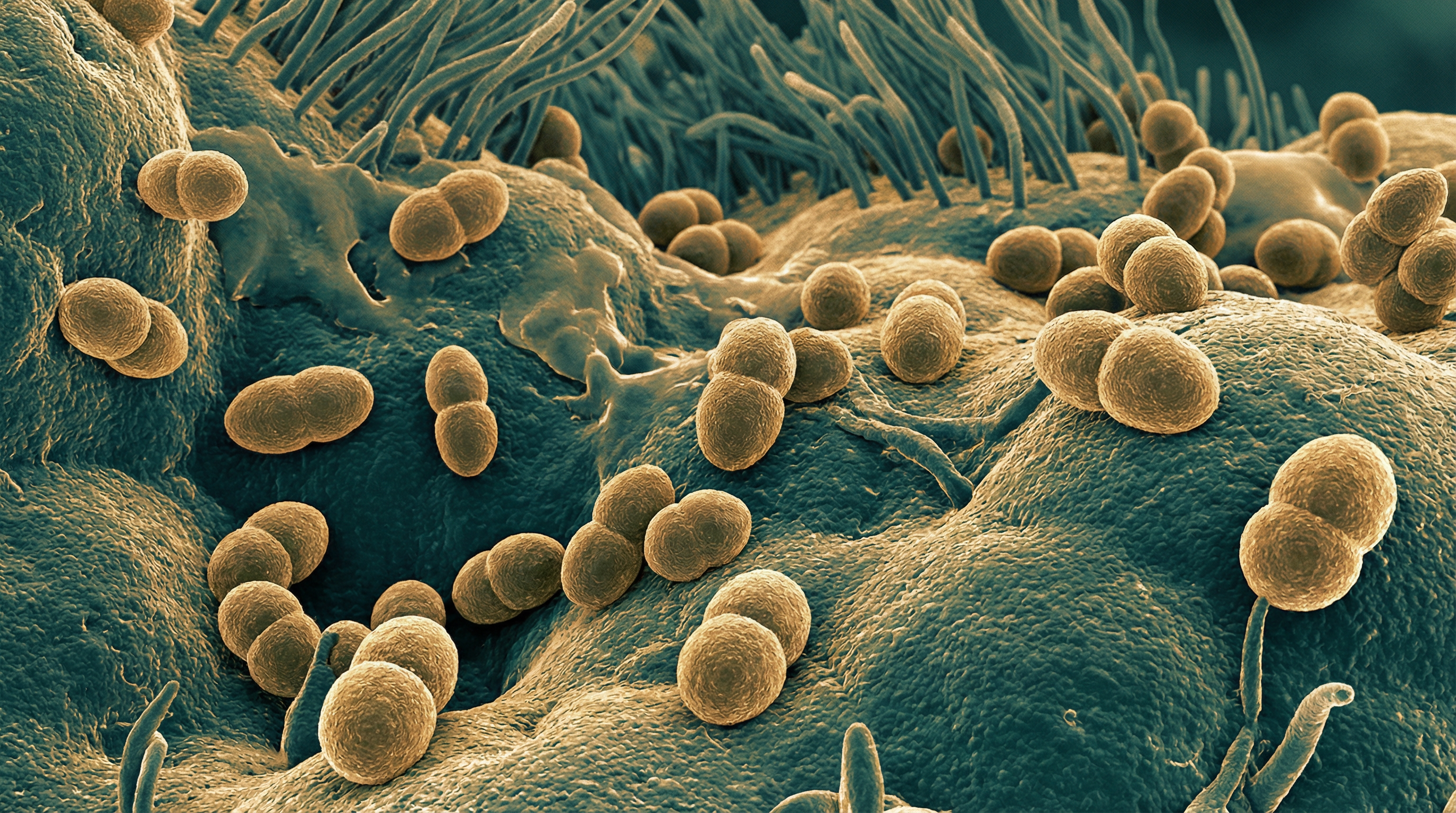
Bordetella pertussis is a Gram-negative, aerobic coccobacillus belonging to the family Alcaligenaceae within the phylum Proteobacteria. It is the primary causative agent of pertussis (whooping cough), a highly contagious respiratory disease. B. pertussis possesses several distinctive characteristics:
- Small (0.2-0.5 μm × 1-2 μm), non-motile, encapsulated coccobacillus
- Strictly aerobic with optimal growth at 35-37°C
- Fastidious organism requiring specialized media for laboratory cultivation
- Slow-growing, typically requiring 3-4 days for visible colony formation
- Forms small, pearl-like, glistening colonies on Bordet-Gengou or Regan-Lowe media
- Produces hemolysis on blood-containing media
- Oxidase-positive but catalase-variable
- Does not ferment carbohydrates and is biochemically relatively inert
- Possesses a complex array of virulence factors regulated by the BvgAS two-component system
- Exhibits antigenic variation through phase variation and antigenic modulation
- Genome size of approximately 4.1 Mb with a relatively high GC content (67%)
- Has undergone significant genome reduction compared to its ancestor, with numerous pseudogenes
- Exclusively human-adapted pathogen with no known animal or environmental reservoir
B. pertussis is part of the Bordetella genus, which includes other respiratory pathogens such as B. parapertussis (causing a milder form of pertussis in humans) and B. bronchiseptica (causing respiratory infections in various animals and occasionally in humans). The genus also includes B. avium and B. hinzii, which primarily cause respiratory disease in birds but can rarely infect humans.
Role in Human Microbiome
B. pertussis is not considered a normal component of the human microbiome. It is a strict human pathogen that transiently colonizes the respiratory tract during infection. Unlike many other respiratory pathogens that can establish asymptomatic carriage, B. pertussis is almost exclusively associated with symptomatic infection, although subclinical infections can occur, particularly in previously vaccinated or infected individuals.
The relationship between B. pertussis and the resident respiratory microbiota is complex:
Impact of respiratory microbiota on B. pertussis colonization:
- The normal respiratory microbiota provides colonization resistance against B. pertussis
- Commensal bacteria compete with B. pertussis for attachment sites on respiratory epithelium
- Some commensal species produce antimicrobial compounds that inhibit B. pertussis growth
- Microbiota-induced host immune responses can limit B. pertussis colonization
- Antibiotic treatment that disrupts the respiratory microbiota can increase susceptibility to B. pertussis infection
Impact of B. pertussis on respiratory microbiota:
- B. pertussis infection causes significant disruption of the respiratory microbiota
- The bacterium's toxins and virulence factors damage ciliated epithelial cells, altering the microenvironment
- Inflammation induced by B. pertussis changes the nutrient availability and oxygen tension in the respiratory tract
- These changes can favor the growth of certain opportunistic pathogens, potentially leading to secondary infections
Interaction with host immune system:
- The respiratory microbiota shapes the development and function of the local immune system
- Pre-existing microbiota-induced immune responses can influence the outcome of B. pertussis infection
- B. pertussis has evolved multiple mechanisms to modulate host immune responses, which may indirectly affect the composition of the respiratory microbiota
Recent research has shown that the composition of the respiratory microbiota may influence the severity and duration of pertussis. Individuals with a more diverse respiratory microbiota may experience less severe disease, suggesting potential for microbiota-based interventions as adjuncts to conventional pertussis prevention and treatment strategies.
Health Implications
B. pertussis infection causes pertussis (whooping cough), a highly contagious respiratory disease with significant global health impact. The health implications of B. pertussis infection include:
Clinical manifestations and disease progression:
- Catarrhal stage (1-2 weeks): Mild upper respiratory symptoms resembling the common cold, including low-grade fever, mild cough, runny nose, and sneezing. This stage is highly contagious.
- Paroxysmal stage (2-6 weeks): Characterized by severe coughing fits (paroxysms) followed by a distinctive high-pitched "whoop" sound during inspiration. Coughing episodes can be accompanied by cyanosis, vomiting, and exhaustion. This stage is most severe and diagnostic.
- Convalescent stage (weeks to months): Gradual resolution of symptoms, though coughing paroxysms may recur with subsequent respiratory infections for up to several months.
Severity spectrum:
- Infants under 6 months: Most severe disease with highest risk of complications and mortality
- Young children: Typically classic whooping cough presentation
- Adolescents and adults: Often atypical presentation with prolonged cough but fewer paroxysms
- Previously vaccinated individuals: Generally milder disease but can still transmit the infection
Complications:
- Respiratory: Pneumonia (primary or secondary bacterial), atelectasis, pneumothorax, subcutaneous emphysema, rib fractures
- Neurological: Seizures, encephalopathy, brain hemorrhage (due to increased intracranial pressure during coughing)
- Other: Weight loss, dehydration, urinary incontinence, rectal prolapse, subconjunctival hemorrhage, hernias
- Fatal complications: Respiratory failure, pulmonary hypertension, cardiac failure (particularly in infants)
Risk factors for severe disease:
- Young age, particularly infants under 6 months
- Lack of vaccination or incomplete vaccination
- Immunocompromised status
- Underlying respiratory or cardiac conditions
- Malnutrition
- Lack of maternal antibodies in infants
Epidemiological impact:
- Global burden: Estimated 24.1 million cases and 160,700 deaths annually, primarily in children under 5 years
- Resurgence in many developed countries despite high vaccination coverage
- Cyclical epidemic pattern with peaks every 3-5 years
- Significant economic burden due to healthcare costs and lost productivity
Long-term sequelae:
- Persistent cough for months after infection
- Increased bronchial hyperreactivity
- Possible link to development of asthma or chronic lung disease
- Neurological sequelae in those who experienced encephalopathy
The health impact of B. pertussis extends beyond the infected individual, as the highly contagious nature of the disease poses a significant risk to vulnerable populations, particularly unvaccinated or incompletely vaccinated infants who are at highest risk for severe disease and death.
Metabolic Activities
B. pertussis exhibits unique metabolic characteristics that reflect its adaptation to the human respiratory tract and its evolution as an obligate human pathogen. Key aspects of its metabolism include:
Carbon metabolism:
- Lacks a complete glycolysis pathway and cannot utilize sugars as carbon sources
- Relies primarily on amino acids as carbon and energy sources
- Preferentially utilizes glutamate, which is the main carbon source for growth
- Can also metabolize alanine and proline efficiently
- Prefers amino acids that are degraded to α-ketoglutarate or pyruvate
- Has undergone significant genome reduction, losing many metabolic pathways present in its ancestors
TCA cycle and energy production:
- Possesses a complete and functional TCA cycle, contrary to earlier beliefs
- Contains genes encoding citrate synthase, aconitase, and isocitrate dehydrogenase
- Cannot utilize citrate as a carbon source despite having the necessary enzymes
- Relies heavily on oxidative phosphorylation for energy production
- Has a high demand for oxygen as a terminal electron acceptor
- Produces ATP primarily through aerobic respiration
Nitrogen metabolism:
- Utilizes amino acids as nitrogen sources
- Can produce excess ammonia during amino acid catabolism, which can inhibit growth at high concentrations
- Requires careful balancing of N:C ratios for optimal growth
- Possesses limited capacity for nitrogen assimilation compared to related species
Lipid metabolism:
- Produces and accumulates poly-hydroxybutyrate as a carbon storage compound
- Releases free fatty acids during growth, which can be inhibitory
- Has limited capacity for fatty acid degradation
- Requires cyclodextrins (like heptakis) in culture media to absorb inhibitory fatty acids
Nutrient requirements:
- Highly fastidious with complex nutritional requirements
- Requires specific amino acids, particularly glutamate and cysteine
- Needs nicotinamide (niacin) as it lacks the ability to synthesize NAD+
- Requires iron, which it acquires using siderophores
- Needs various vitamins and growth factors
- Cannot synthesize many essential compounds due to genome reduction
Metabolic adaptation to host environment:
- Adapted to utilize nutrients available in the respiratory tract
- Metabolism is tightly regulated by the BvgAS two-component system, which also controls virulence
- Metabolic genes are co-regulated with virulence factors
- Can adjust metabolism in response to environmental signals like temperature and nutrient availability
- Metabolic activities are linked to virulence, with certain metabolites serving as signals for virulence gene expression
Growth characteristics:
- Relatively slow growth rate compared to many other bacteria
- Low final biomass yields in culture
- Growth inhibited by high substrate concentrations
- Sensitive to changes in pH and salt concentrations
- Requires specific environmental conditions for optimal growth
These metabolic characteristics have important implications for both the pathogenesis of B. pertussis and for vaccine production, which requires growing large quantities of the bacterium. Understanding and optimizing B. pertussis metabolism is crucial for improving vaccine manufacturing processes and potentially for developing new therapeutic approaches.
Clinical Relevance
B. pertussis remains a significant global health concern despite the availability of vaccines. Its clinical relevance encompasses several aspects:
Diagnosis:
- Clinical diagnosis: Based on characteristic paroxysmal cough with inspiratory "whoop," post-tussive vomiting, and prolonged cough duration
- Laboratory confirmation:
- Culture: Gold standard but slow (3-7 days) and sensitivity decreases with disease duration and antibiotic use
- PCR: Rapid and sensitive, especially early in disease; can detect non-viable organisms
- Serology: Useful in later stages when culture and PCR sensitivity decreases; measures antibodies to pertussis toxin
- Direct fluorescent antibody (DFA) testing: Rapid but less sensitive and specific
- Diagnostic challenges:
- Atypical presentation in infants, vaccinated individuals, and adults
- Reduced sensitivity of tests in later disease stages
- Difficulty distinguishing from other causes of prolonged cough
- Limited availability of diagnostic tests in resource-limited settings
Treatment:
- Antimicrobial therapy:
- Macrolides (azithromycin, clarithromycin, erythromycin) are first-line treatments
- Trimethoprim-sulfamethoxazole as an alternative for macrolide-allergic patients
- Most effective when initiated early in the catarrhal stage
- Primarily reduces infectiousness rather than altering disease course if started after catarrhal stage
- Emerging macrolide resistance is a concern in some regions
- Supportive care:
- Hospitalization for severe cases, particularly in infants
- Respiratory support ranging from supplemental oxygen to mechanical ventilation
- Careful fluid management and nutritional support
- Monitoring for complications
- Avoidance of antitussives, which are ineffective and potentially harmful
- Antimicrobial therapy:
Prevention:
- Vaccination:
- Primary prevention strategy
- Two main types: whole-cell (wP) and acellular (aP) vaccines
- wP vaccines: More reactogenic but potentially more effective long-term
- aP vaccines: Better safety profile but shorter duration of protection
- Recommended schedule includes primary series in infancy and booster doses
- Maternal vaccination during pregnancy provides passive immunity to newborns
- Post-exposure prophylaxis:
- Antimicrobial prophylaxis for close contacts of confirmed cases
- Particularly important for protecting vulnerable individuals
- Infection control measures:
- Droplet precautions for hospitalized patients
- Exclusion of infected individuals from school/work during infectious period
- Vaccination:
Public health significance:
- Resurgence in vaccinated populations:
- Increasing incidence in countries with high vaccination coverage
- Attributed to waning immunity, vaccine effectiveness, and pathogen adaptation
- Shift in age distribution toward adolescents and adults
- Surveillance challenges:
- Underdiagnosis and underreporting, particularly in adults
- Variable case definitions across different surveillance systems
- Limited surveillance capacity in many low and middle-income countries
- Outbreak management:
- Rapid identification and treatment of cases
- Contact tracing and prophylaxis
- Targeted vaccination campaigns
- Public education about symptoms and transmission
- Resurgence in vaccinated populations:
Emerging concerns:
- Genetic evolution of B. pertussis:
- Emergence of strains with altered antigenic profiles
- Pertactin-deficient strains becoming prevalent in some regions
- Potential impact on vaccine effectiveness
- Antimicrobial resistance:
- Increasing reports of macrolide resistance
- Limited alternative treatment options
- Vaccine hesitancy:
- Declining vaccination rates in some populations
- Increased risk of outbreaks and infant exposure
- Genetic evolution of B. pertussis:
The clinical management of pertussis remains challenging, particularly in young infants where the disease can be life-threatening. Continued research on improved diagnostics, treatment approaches, and vaccination strategies is essential for effective control of this disease.
Interaction with Other Microorganisms
B. pertussis interacts with other microorganisms primarily within (Content truncated due to size limit. Use line ranges to read in chunks)
