Borrelia burgdorferi
Key Characteristics
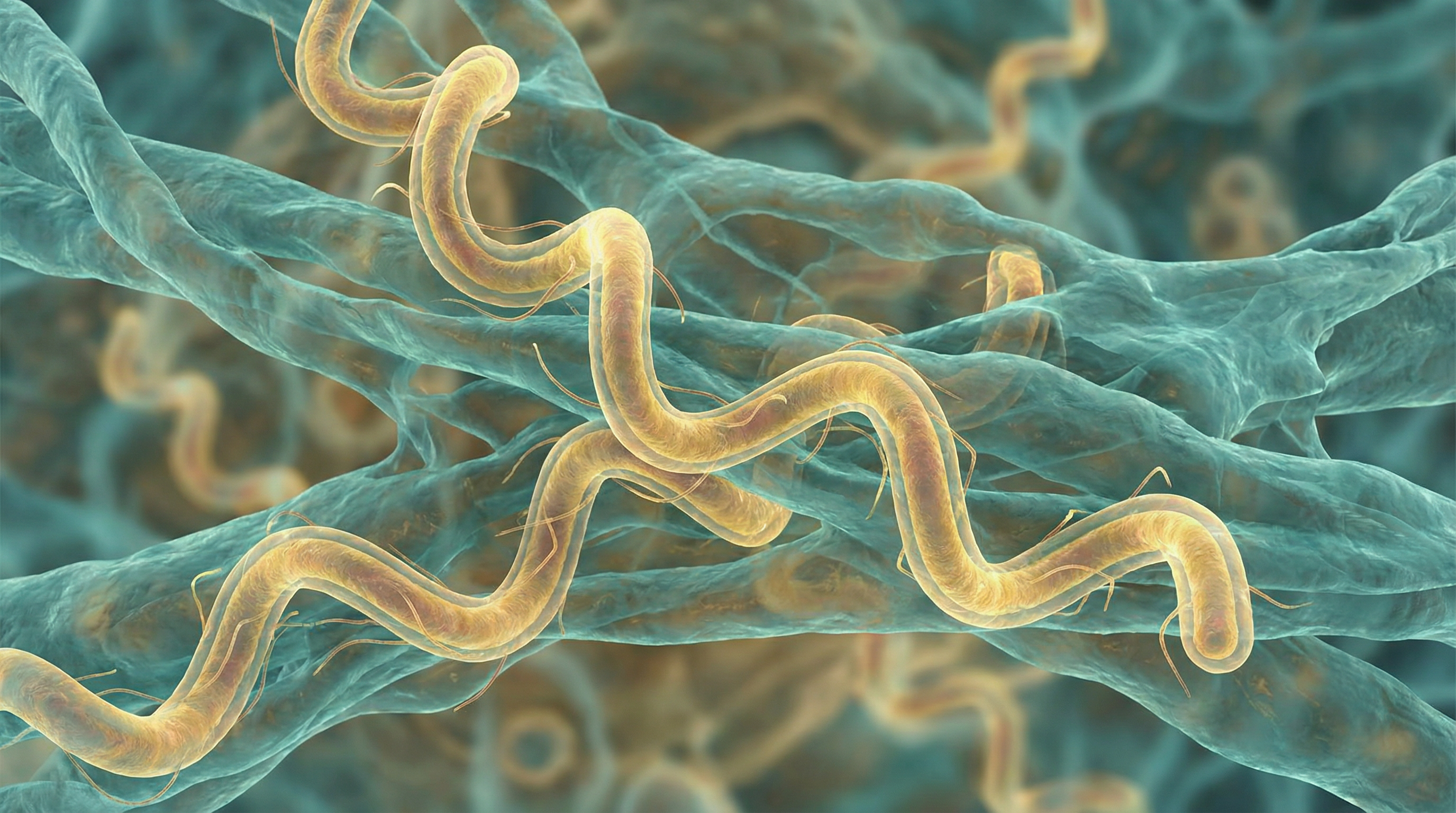
Borrelia burgdorferi is a gram-negative, microaerophilic, spiral-shaped bacterium belonging to the phylum Spirochaetes. It is the primary causative agent of Lyme disease in North America, while related species (B. afzelii and B. garinii) cause the disease in Europe and Asia. B. burgdorferi possesses several distinctive characteristics:
- Spiral-shaped (spirochete) morphology with a length of 10-30 μm and width of 0.2-0.5 μm
- Highly motile with unique endoflagella (7-11 flagella) that wrap around the cell cylinder between the outer membrane and peptidoglycan layer
- Corkscrew-like motility that enables tissue penetration and immune evasion
- Unusual cell wall structure containing chitobiose, a component derived from its tick vector
- Complex genome consisting of a linear chromosome (~950 kb) and numerous linear and circular plasmids (up to 21 plasmids)
- Lacks genes for lipopolysaccharide synthesis, toxin production, and many metabolic pathways
- Obligate parasite that requires a tick vector and vertebrate host to complete its life cycle
- Extremely limited metabolic capabilities, relying heavily on host nutrients
- Optimal growth temperature of 33-37°C, reflecting adaptation to both tick and mammalian hosts
- Slow generation time of 12-24 hours, much longer than most bacteria
B. burgdorferi is transmitted to humans through the bite of infected Ixodes ticks (primarily I. scapularis in the eastern and midwestern United States, I. pacificus in the western United States, and I. ricinus in Europe). The bacterium has evolved sophisticated mechanisms to survive in both arthropod and mammalian environments, making it a highly successful pathogen.
Role in Human Microbiome
Unlike many other bacteria in the human microbiome, B. burgdorferi is not considered a normal commensal organism. It functions exclusively as a pathogen in humans, who are incidental hosts in its natural transmission cycle. The bacterium's primary ecological niche involves cycling between Ixodes ticks and small mammal reservoir hosts, particularly rodents like the white-footed mouse (Peromyscus leucopus) in North America.
When introduced into human skin through a tick bite, B. burgdorferi interacts with the resident skin microbiota and host immune cells. Research suggests that the composition of the skin microbiome may influence the initial establishment of B. burgdorferi infection, with certain commensal bacteria potentially providing either protective or facilitating effects.
B. burgdorferi has evolved specialized mechanisms to survive within the human host, including:
- Ability to adhere to host cells and extracellular matrix components
- Capacity to penetrate tissues and disseminate throughout the body via the bloodstream
- Remarkable immune evasion strategies, including antigenic variation, complement inhibition, and modulation of host immune responses
- Ability to establish persistent infection in immunoprivileged sites
Unlike many other bacterial pathogens, B. burgdorferi does not produce toxins or cause direct tissue damage. Instead, most of the pathology associated with Lyme disease results from the host's inflammatory response to the bacterium.
Health Implications
B. burgdorferi causes Lyme disease, the most common vector-borne disease in the United States with approximately 476,000 Americans diagnosed and treated annually according to CDC estimates. The disease typically progresses through several stages if left untreated:
Early Localized Disease (3-30 days post-tick bite): Characterized by erythema migrans (EM), a bull's-eye rash that appears at the site of the tick bite in approximately 70-80% of infected individuals. Other symptoms may include fever, fatigue, headache, muscle and joint pain, and swollen lymph nodes.
Early Disseminated Disease (days to weeks post-infection): As the bacteria spread through the bloodstream, multiple EM lesions may appear at sites distant from the tick bite. Neurological symptoms (facial palsy, meningitis, radiculoneuritis), cardiac abnormalities (atrioventricular heart block), and musculoskeletal pain can develop.
Late Disease (months to years post-infection): Characterized by intermittent or persistent arthritis primarily affecting large joints, especially the knee. Chronic neurological manifestations may include encephalopathy, polyneuropathy, and cognitive difficulties.
The health impact of B. burgdorferi infection varies widely among individuals, with some experiencing mild, self-limiting symptoms and others developing severe, chronic manifestations. Factors influencing disease severity include the bacterial strain, host genetic factors, immune response, and timing of antibiotic treatment.
Importantly, B. burgdorferi infection in its natural reservoir hosts (rodents) is typically asymptomatic or causes mild disease, suggesting co-evolution between the pathogen and these hosts. In contrast, humans and other incidental hosts often experience significant inflammatory disease, indicating a less well-adapted host-pathogen relationship.
Metabolic Activities
B. burgdorferi has extremely limited metabolic capabilities compared to most bacteria, which reflects its evolution as an obligate parasite. Key metabolic features include:
- Lacks genes for the tricarboxylic acid (TCA) cycle, oxidative phosphorylation, and electron transport chains
- Relies on glycolysis as the primary energy-generating pathway
- Cannot synthesize amino acids, nucleotides, fatty acids, or enzyme cofactors
- Possesses transport systems to acquire essential nutrients from the host
- Utilizes glucose as the preferred carbon source, but can also metabolize glycerol, chitobiose, N-acetylglucosamine, and maltose
- Requires long-chain fatty acids from the host for membrane synthesis
- Possesses a unique mevalonate pathway for cell wall synthesis
- Has a distinctive peptidoglycan structure containing chitobiose (GlcNAc-GlcNAc), a component derived from its tick vector
- Microaerophilic, requiring small amounts of oxygen for metabolism
The bacterium's limited metabolic repertoire explains its strict dependence on host-derived nutrients and its inability to survive outside of a host environment. This metabolic dependency also makes B. burgdorferi particularly vulnerable to environmental stresses and antimicrobial agents that target its few essential metabolic pathways.
Interestingly, B. burgdorferi can manipulate host cell metabolism to create a more favorable environment for its survival. Recent research has shown that the bacterium can induce metabolic changes in immune cells, potentially contributing to immune evasion and persistence.
Clinical Relevance
B. burgdorferi infection remains a significant public health concern, particularly in endemic regions of North America, Europe, and Asia. Clinical relevance includes:
Diagnosis: Relies on a two-tier testing approach:
- Initial screening with enzyme immunoassay (EIA) or immunofluorescence assay (IFA)
- Confirmation of positive or equivocal results with Western blot
- Modified two-tier testing using two different EIAs is now also recommended
- Direct detection methods (PCR, culture) have limited sensitivity in clinical practice
- Clinical manifestations and history of tick exposure are crucial diagnostic considerations
Treatment: Antibiotic therapy is effective, especially when initiated early:
- Early localized and early disseminated disease: Doxycycline, amoxicillin, or cefuroxime for 10-21 days
- Neurological manifestations: Intravenous ceftriaxone, cefotaxime, or penicillin G for 14-28 days
- Lyme arthritis: Oral antibiotics for 28 days; persistent arthritis may require intravenous therapy
- Post-treatment Lyme disease syndrome (PTLDS): Prolonged antibiotic therapy not recommended
Prevention: Strategies include:
- Tick avoidance and prompt removal
- Use of repellents and protective clothing
- Environmental modifications to reduce tick habitats
- Vaccination (currently only available for dogs in the United States)
Public Health Significance: Lyme disease incidence has increased significantly in recent decades, with geographic expansion into previously non-endemic areas. Climate change, land use changes, and increasing deer and rodent populations have contributed to this trend.
Controversies: Persistent controversy exists regarding chronic Lyme disease, with some patients reporting persistent symptoms despite standard antibiotic treatment. The medical community remains divided on the etiology of these symptoms and appropriate management approaches.
Interaction with Other Microorganisms
B. burgdorferi's interactions with other microorganisms occur primarily in two distinct environments: the tick vector and the mammalian host. These interactions include:
Interactions in the tick microbiome:
- Co-exists with other tick-borne pathogens such as Anaplasma phagocytophilum, Babesia microti, and Powassan virus
- Co-infection with these pathogens can alter disease manifestations and severity
- Competes with and is influenced by the resident tick microbiota
- Some tick microbiome members may inhibit B. burgdorferi colonization, while others may facilitate it
Interactions in the mammalian host:
- Interacts with the skin microbiome at the site of tick bite
- Certain skin commensals may provide protection against B. burgdorferi establishment
- Disruption of the normal skin microbiota may facilitate B. burgdorferi invasion
- Systemic dissemination brings B. burgdorferi into contact with microbiota at various body sites
Immune modulation:
- B. burgdorferi can manipulate host immune responses, potentially altering the host's relationship with its commensal microbiota
- Infection may lead to dysbiosis in certain body sites, contributing to disease manifestations
- The bacterium's immune evasion strategies may create niches for opportunistic pathogens
Biofilm associations:
- Some research suggests B. burgdorferi may form biofilm-like structures in certain environments
- These structures could provide protection against antibiotics and host immune responses
- Potential interactions with other biofilm-forming bacteria remain an area of active investigation
The complex interactions between B. burgdorferi, other microorganisms, and the host immune system likely contribute to the variable clinical manifestations and outcomes of Lyme disease. Understanding these interactions may provide insights into novel prevention and treatment strategies.
Research Significance
B. burgdorferi has significant research importance for several reasons:
Model for vector-borne diseases: The B. burgdorferi transmission cycle serves as an important model for understanding the ecology and epidemiology of vector-borne diseases, particularly in the context of climate change and expanding geographic ranges.
Immune evasion mechanisms: The bacterium's sophisticated strategies for evading host immune responses provide valuable insights into host-pathogen interactions and bacterial persistence.
Unique biology: Several aspects of B. burgdorferi biology are unusual, including its segmented genome, distinctive cell wall structure, limited metabolic capabilities, and specialized motility mechanisms.
Public health significance: As the causative agent of the most common vector-borne disease in the United States and a significant health concern in Europe and Asia, B. burgdorferi research has direct implications for public health policy and clinical practice.
Controversial aspects: The ongoing controversy regarding chronic Lyme disease highlights the need for continued research into bacterial persistence, host factors influencing disease manifestations, and optimal treatment approaches.
Recent research directions include:
- Molecular mechanisms of B. burgdorferi pathogenesis and immune evasion
- Development of improved diagnostic tests, particularly for early disease
- Investigation of factors contributing to post-treatment symptoms
- Exploration of novel vaccine candidates
- Understanding the impact of climate change on B. burgdorferi ecology and epidemiology
- Characterization of the unique cell wall structure and its role in pathogenesis
- Elucidation of the genetic basis for strain-specific differences in virulence and tissue tropism
Advances in genomics, proteomics, and imaging technologies have accelerated B. burgdorferi research in recent years, providing new insights into this complex pathogen and the disease it causes.
References
Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10(2):87-99.
Coburn J, Garcia B, Hu LT, et al. Lyme Disease Pathogenesis. Curr Issues Mol Biol. 2021;42:473-518.
DeHart TG, Kushelman MR, Hildreth SB, Helm RF, Jutras BL. The unusual cell wall of the Lyme disease spirochaete Borrelia burgdorferi is shaped by a tick sugar. Nat Microbiol. 2021;6(12):1583-1592.
Tracy KE, Baumgarth N. Borrelia burgdorferi Manipulates Innate and Adaptive Immunity to Establish Persistence in Rodent Reservoir Hosts. Front Immunol. 2017;8:116.
Tatum R, Pearson-Shaver AL. Borrelia Burgdorferi. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
Hyde JA. Borrelia burgdorferi Keeps Moving and Carries on: A Review of Borrelial Dissemination and Invasion. Front Immunol. 2017;8:114.
Steere AC, Strle F, Wormser GP, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090.
Brissette CA, Gaultney RA. That's my story, and I'm sticking to it--an update on B. burgdorferi adhesins. Front Cell Infect Microbiol. 2014;4:41.
