Candida albicans
Overview
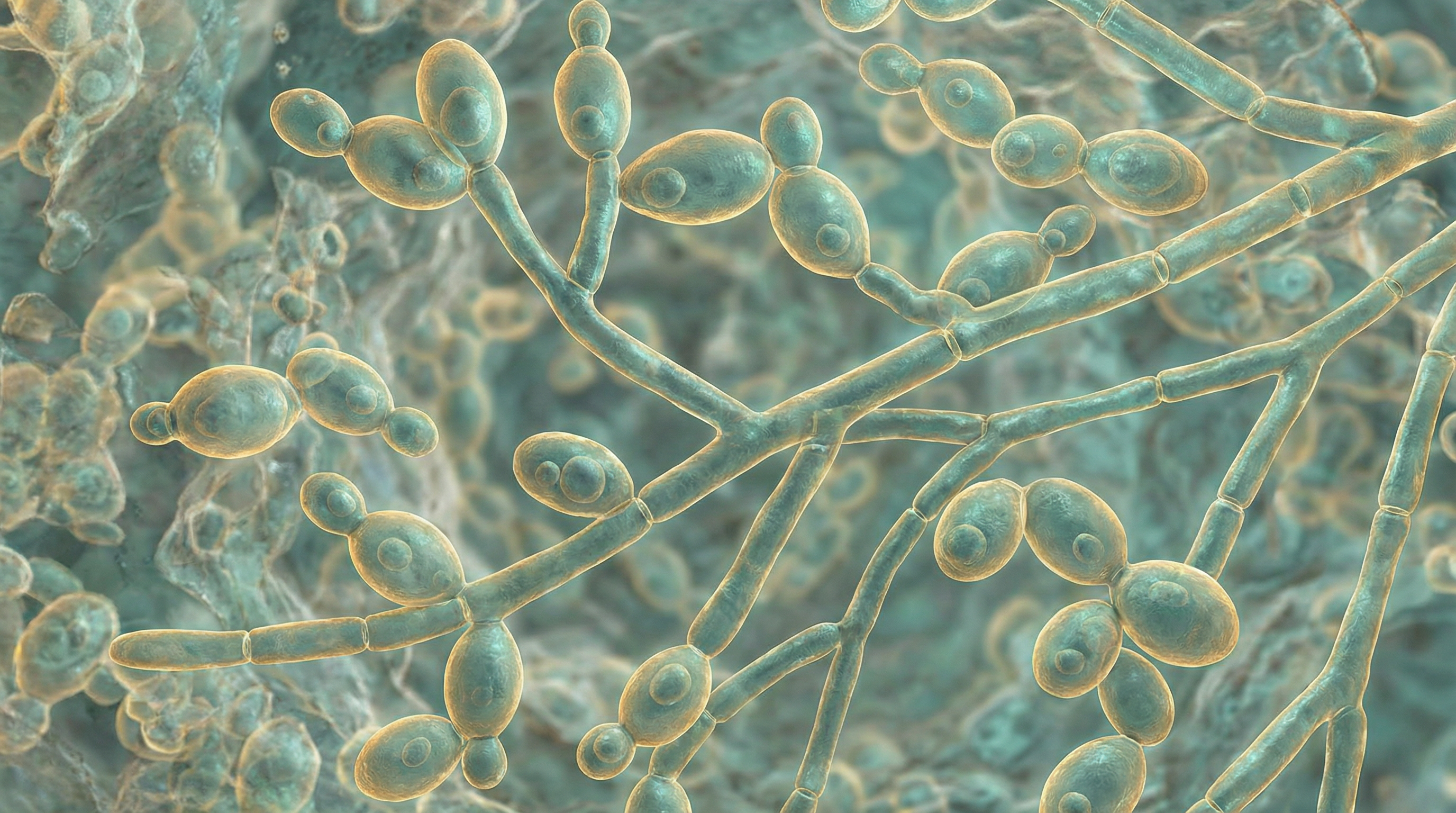
Candida albicans is a dimorphic fungus that exists as a common commensal organism in approximately 50% of the human population. It naturally colonizes the oropharyngeal cavity, gastrointestinal tract, vaginal tract, and skin without causing disease in healthy individuals. As the most prevalent fungal species in the human gut microbiome, C. albicans has coevolved with humans and appears to have no major environmental reservoir outside the human body. This close evolutionary relationship has resulted in a complex interplay between the fungus and its human host.
While C. albicans typically exists as a harmless commensal, it is also an opportunistic pathogen capable of causing a spectrum of conditions ranging from superficial mucosal infections to life-threatening systemic diseases. The transition from commensal to pathogen occurs when there are disturbances in the normal homeostasis of the host, such as immune dysfunction, disruption of the microbiota, or damage to mucosal barriers. This transition is facilitated by numerous virulence factors that C. albicans possesses, including its ability to switch between different morphological forms, adhere to host tissues, produce hydrolytic enzymes, and form biofilms.
The relationship between C. albicans and the human host is maintained in balance by the immune system, which recognizes and controls the fungus without eliminating it completely. This balance allows C. albicans to persist as part of the normal microbiota while preventing pathogenic overgrowth. The interactions between C. albicans and other members of the microbiota, particularly bacteria, further influence this balance and contribute to maintaining homeostasis in the gut and other body sites.
Understanding the dual nature of C. albicans as both a commensal and pathogen is essential for comprehending its role in human health and disease. Research into the factors that govern the transition between these states continues to provide insights into fungal pathogenesis and may lead to novel approaches for preventing and treating Candida infections.
Characteristics
Candida albicans exhibits several distinctive characteristics that define its biological identity and contribute to its ability to thrive as both a commensal organism and an opportunistic pathogen:
Morphological Versatility:
- Exists in multiple morphological forms: blastospores (yeast), pseudohyphae, and true hyphae
- Capable of reversible morphological transitions in response to environmental cues
- Blastospores are oval-shaped cells that reproduce by budding
- Pseudohyphae are chains of elongated yeast cells
- Hyphae are branched chains of tubular cells without narrowing at septation sites
- Can also form chlamydospores (thick-walled, resistant cells) under adverse conditions
Cell Structure:
- Cell wall composed of glucan, chitin, and proteins
- Cell wall provides protection against environmental stressors and host immune defenses
- Contains adhesion proteins (Als1-7, Als9, Hwp1) that facilitate attachment to host tissues
- Cell membrane contains ergosterol, a fungal-specific sterol that provides stability and rigidity
- Phospholipid bilayer contains proteins that function as receptors, transporters, and signal transducers
Genetic and Genomic Features:
- Diploid organism with significant genetic plasticity
- Genome size of approximately 14.4 megabases
- Contains approximately 6,000 genes
- Exhibits natural heterozygosity at many genetic loci
- Capable of undergoing parasexual reproduction, contributing to genetic diversity
- Can undergo chromosomal rearrangements and aneuploidy in response to stress
Metabolic Capabilities:
- Utilizes glucose as primary carbon source
- Can metabolize various amino acids as nitrogen sources
- Adaptable metabolism that can adjust to different nutrient environments
- Capable of both fermentative and respiratory metabolism
- Can grow under both aerobic and anaerobic conditions
- Possesses metabolic flexibility that allows survival in diverse host niches
Growth Requirements:
- Optimal growth temperature around 37°C (human body temperature)
- Prefers neutral to slightly acidic pH
- Growth enhanced by high CO2 concentrations
- Can grow in environments with limited nutrients
- Filamentation (hyphal formation) enhanced by temperature >37°C, alkaline pH, serum, and high CO2
Virulence Factors:
- Morphological transition from yeast to hyphal forms
- Production of candidalysin, a hypha-specific cytolytic toxin
- Expression of adhesins that facilitate attachment to host surfaces
- Secretion of hydrolytic enzymes (proteases, phospholipases, lipases)
- Formation of biofilms on biotic and abiotic surfaces
- Phenotypic switching between different colony types
- Ability to evade host immune responses
Ecological Distribution:
- Primarily associated with humans and other warm-blooded animals
- Most abundant fungus in the human gut microbiome
- Common colonizer of oral cavity, gastrointestinal tract, vaginal tract, and skin
- Present in approximately 50% of healthy individuals
- No significant environmental reservoir outside the human body
- Vertical transmission from mother to infant during birth
Antimicrobial Susceptibility:
- Naturally susceptible to most antifungal agents
- Can develop resistance through various mechanisms
- Target of azole antifungals through ergosterol biosynthesis pathway
- Susceptible to polyenes that bind to membrane ergosterol
- Can be inhibited by echinocandins that target cell wall synthesis
- Capable of forming biofilms that confer increased resistance to antifungals
These characteristics collectively enable C. albicans to successfully colonize various human body sites, interact with the host and other microorganisms, and transition between commensal and pathogenic states in response to changes in the host environment. The remarkable adaptability of C. albicans, particularly its morphological plasticity and metabolic flexibility, contributes significantly to its success as both a commensal organism and an opportunistic pathogen.
Role in Human Microbiome
Candida albicans occupies a significant niche within the human microbiome, with complex interactions that influence both host health and microbial community dynamics:
Distribution and Prevalence:
- Present in approximately 50% of healthy individuals
- Most abundant fungal species in the human gut microbiome
- Common colonizer of the oral cavity, particularly the tongue and palate
- Frequently found in the vaginal tract, where it can comprise part of the normal flora
- Can colonize skin, especially in moist, warm areas
- Typically maintained at low levels in healthy individuals due to competition with bacteria and host immune surveillance
Ecological Niche:
- Occupies specific microhabitats within the body based on local conditions
- In the gut, primarily resides in the lower gastrointestinal tract
- Forms part of the "mycobiome," the fungal component of the human microbiome
- Population density varies by body site, with highest numbers typically in the colon
- Colonization begins early in life, often acquired during birth from maternal vaginal flora
- Persistent colonizer that can remain in the same host for years
Interactions with Bacterial Microbiota:
- Engages in complex bidirectional relationships with bacterial community members
- Competition with bacteria for nutrients and attachment sites helps maintain fungal homeostasis
- Certain bacteria (e.g., lactobacilli) inhibit C. albicans growth through production of organic acids and antimicrobial compounds
- Other bacteria may promote C. albicans colonization by providing nutrients or creating favorable microenvironments
- Antibiotic treatment disrupts bacterial communities, often leading to C. albicans overgrowth
- C. albicans can influence bacterial community composition and reassembly after perturbations
Metabolic Contributions:
- Participates in carbohydrate metabolism within the gut ecosystem
- May contribute to breakdown of complex dietary components
- Produces metabolites that can influence both host cells and other microorganisms
- Capable of utilizing byproducts of bacterial metabolism
- Adapts its metabolism based on available nutrients and oxygen levels in different body sites
- May participate in cross-feeding relationships with certain bacterial species
Biofilm Formation:
- Forms mixed-species biofilms with bacteria in various body sites
- Biofilm formation contributes to stable colonization and persistence
- Biofilms provide protection against host defenses and antimicrobial agents
- Mixed fungal-bacterial biofilms can exhibit enhanced resistance properties
- Biofilm architecture creates microenvironments that support diverse microbial populations
- Communication between species within biofilms occurs through various signaling molecules
Host-Microbe Interactions:
- Recognized by the host immune system but typically tolerated as a commensal
- Induces T helper 17 (Th17) cell responses, which are central orchestrators of protective immunity
- Maintains a balance with host defenses that allows persistent colonization without disease
- Interacts with host epithelial cells through various adhesins and receptors
- Stimulates production of antimicrobial peptides that help regulate microbial communities
- Contributes to training and development of the host immune system
Response to Environmental Changes:
- Population levels fluctuate in response to diet, host factors, and changes in bacterial communities
- Increases in abundance following antibiotic treatment due to reduced bacterial competition
- Responds to changes in host hormone levels, particularly in the vaginal environment
- Adapts to variations in pH, oxygen levels, and nutrient availability across different body sites
- Can undergo ecological succession during development and aging of the host
- Demonstrates resilience, often returning to baseline levels after temporary perturbations
Dysbiosis and Disease Association:
- Disruption of normal fungal-bacterial balance can lead to C. albicans overgrowth
- Fungal dysbiosis may contribute to inflammatory conditions and altered immune responses
- Changes in C. albicans abundance have been associated with various gastrointestinal disorders
- Shifts in the mycobiome may influence bacterial dysbiosis and vice versa
- Restoration of normal microbial communities can help resolve C. albicans overgrowth
- Emerging evidence suggests potential roles in metabolic disorders and neurological conditions
The role of C. albicans in the human microbiome represents a delicate balance between commensalism and potential pathogenicity. As research continues to elucidate the complex interactions between fungi, bacteria, and the host, a more comprehensive understanding of C. albicans' contribution to microbiome function and host health is emerging. This knowledge may lead to novel approaches for maintaining healthy microbial communities and preventing Candida-associated diseases.
Health Implications
Candida albicans has significant health implications that span from beneficial immunomodulatory effects to potentially life-threatening infections:
Commensal Relationship:
- In healthy individuals, C. albicans exists as a harmless commensal
- Contributes to training and development of the host immune system
- Induces T helper 17 (Th17) cell responses that provide protection against various pathogens
- May help maintain microbial diversity by competing with potentially harmful microorganisms
- Commensal colonization may provide colonization resistance against more virulent Candida species
- This balanced relationship persists as long as host defenses and microbial communities remain intact
Mucosal Infections:
- Oral Candidiasis (Thrush): White, cottage cheese-like lesions on oral mucosa, tongue, and palate
- Vulvovaginal Candidiasis: Affects up to 75% of women at least once in their lifetime, causing itching, burning, and discharge
- Esophageal Candidiasis: Painful swallowing, retrosternal pain, and potential for bleeding
- Gastrointestinal Candidiasis: Abdominal pain, bloating, and altered bowel habits
- Mucosal infections typically occur when local defenses are compromised or microbial communities are disrupted
- Generally respond well to topical or oral antifungal treatments
Cutaneous Infections:
- Diaper Dermatitis: Common in infants, presenting as bright red rash in the diaper area
- Intertrigo: Infection in skin folds where moisture accumulates
- Onychomycosis: Nail infection, though C. albicans is less common than dermatophytes in this condition
- Paronychia: Infection of the tissue surrounding the nail
- Skin infections often occur in areas with moisture, occlusion, or frequent friction
- May be exacerbated by diabetes, obesity, or immunosuppression
Invasive Infections:
- Candidemia: Presence of Candida in the bloodstream, with mortality rates approaching 40%
- Disseminated Candidiasis: Spread to multiple organs including kidneys, liver, spleen, and brain
- Endocarditis: Infection of heart valves, particularly in patients with prosthetic valves
- Meningitis: Rare but serious infection of the meninges
- Invasive infections typically occur in severely immunocompromised patients or those with multiple risk factors
- Require prompt systemic antifungal therapy and often source control
Risk Factors for Infection:
- Immunosuppression: HIV/AIDS, chemotherapy, transplant recipients, corticosteroid use
- Antibiotic Use: Disrupts bacterial communities that normally suppress C. albicans
- Medical Devices: Catheters, prosthetic joints, heart valves, and other implanted devices
- Diabetes Mellitus: Elevated blood glucose creates favorable conditions for fungal growth
- Extremes of Age: Neonates and elderly individuals have higher susceptibility
- Disrupted Barriers: Burns, wounds, surgery, or mucosal damage
- Pregnancy: Hormonal changes can promote vaginal candidiasis
Chronic Conditions and Controversies:
- Recurrent Vulvovaginal Candidiasis: Affects 5-8% of women with four or more episodes annually
- Chronic Mucocutaneous Candidiasis: Persistent infections associated with specific immune defects
- "Candida Hypersensitivity Syndrome": Controversial condition attributed to systemic candidiasis without scientific consensus
- Gut Dysbiosis: Potential role in irritable bowel syndrome and inflammatory bowel disease, though causal relationships remain unclear
- Research continues on potential associations with various autoimmune and inflammatory conditions
Immune Responses and Inflammation:
- C. albicans stimulates both innate and adaptive immune responses
- Recognition by pattern recognition receptors triggers inflammatory cascades
- Excessive inflammation can contribute to tissue damage during infection
- Chronic colonization may influence systemic inflammatory markers
- Emerging evidence suggests potential roles in inflammatory bowel diseases and other inflammatory conditions
- Balance between protective immunity and harmful inflammation is crucial
Therapeutic Considerations:
- Antifungal Resistance: Increasing concern, particularly with azole antifungals
- Biofilm Formation: Reduces effectiveness of antifungal treatments
- Probiotic Approaches: Lactobacillus species may help prevent and treat certain Candida infections
- Dietary Factors: Some evidence suggests high sugar diets may promote Candida overgrowth
- Microbiome Restoration: Fecal microbiota transplantation being investigated for recurrent infections
- Immunotherapy: Approaches targeting host-fungal interface under development
The health implications of C. albicans highlight its dual nature as both a commensal organism and an opportunistic pathogen. Understanding the factors that govern the transition between these states is crucial for developing effective strategies to prevent and treat Candida infections while preserving the potential benefits of commensal colonization. As research continues to elucidate the complex interactions between C. albicans, the host, and other microorganisms, new approaches to managing Candida-related health issues are likely to emerge.
Metabolic Activities
Candida albicans exhibits diverse metabolic capabilities that enable it to adapt to various host environments and contribute to its success as both a commensal organism and an opportunistic pathogen:
Carbon Metabolism:
- Preferentially utilizes glucose through glycolysis and fermentation
- Can switch between fermentative and respiratory metabolism depending on oxygen availability
- Capable of utilizing alternative carbon sources when glucose is limited:
- Other hexoses (fructose, mannose, galactose)
- Non-fermentable carbon sources (glycerol, lactate, ethanol)
- Amino acids and fatty acids
- Possesses specialized pathways for assimilating N-acetylglucosamine (GlcNAc), a component of host tissues
- Regulates carbon metabolism through complex signaling pathways including Ras/cAMP/PKA
- Carbon source availability influences morphological transitions and virulence
Nitrogen Metabolism:
- Utilizes amino acids as preferred nitrogen sources
- Can assimilate ammonium through glutamate dehydrogenase and glutamine synthetase
- Secretes proteases that break down host proteins to release amino acids
- Regulates nitrogen metabolism through nitrogen catabolite repression (NCR)
- Nitrogen limitation can trigger filamentous growth
- Possesses specialized transporters for acquiring nitrogen compounds from host environments
- Adapts nitrogen metabolism based on available resources in different host niches
Lipid Metabolism:
- Synthesizes ergosterol as the major sterol in cell membranes (target of azole antifungals)
- Produces phospholipids for membrane biogenesis
- Can utilize exogenous fatty acids or synthesize them de novo
- Secretes lipases that break down host lipids
- Lipid metabolism influences membrane fluidity and permeability
- Produces prostaglandins that may modulate host immune responses
- Stores excess lipids in lipid droplets during nutrient-rich conditions
Metabolic Adaptation to Host Environments:
- Adjusts metabolism based on nutrient availability in different body sites:
- Glucose-rich environments in oral cavity and vagina
- Amino acid and lipid-rich environments in gastrointestinal tract
- Nutrient-limited conditions during invasive infection
- Modifies metabolic pathways in response to pH changes across host niches
- Adapts to variations in oxygen availability from aerobic to microaerophilic conditions
- Upregulates stress response pathways during nutrient limitation or host defense encounters
- Metabolic flexibility contributes to successful colonization of diverse host environments
- Adjusts metabolism based on nutrient availability in different body sites:
Biofilm Metabolism:
- Exhibits distinct metabolic profiles in biofilm versus planktonic growth
- Creates metabolic heterogeneity within biofilm structures
- Lower metabolic activity in biofilm cells contributes to antifungal resistance
- Utilizes alternative carbon sources in mature biofilms
- Engages in metabolic cooperation with bacterial species in mixed biofilms
- Produces extracellular matrix components through specialized metabolic pathways
- Biofilm formation alters expression of metabolic genes
Metabolic Interactions with Host and Microbiota:
- Competes with gut bacteria for dietary carbohydrates
- May utilize byproducts of bacterial metabolism (e.g., short-chain fatty acids)
- Produces metabolites that can influence bacterial growth and host responses
- Responds to metabolic signals from host cells and other microorganisms
- Adapts metabolism in response to host-derived antimicrobial compounds
- Metabolic activities can influence local microenvironments (e.g., pH, oxygen levels)
- Potential for metabolic cross-feeding relationships with specific bacterial species
Metabolic Regulation of Virulence:
- Carbon source sensing linked to hyphal morphogenesis through cAMP/PKA pathway
- GlcNAc metabolism triggers hyphal growth and expression of virulence genes
- Amino acid sensing through general control nonderepressible 4 (GCN4) influences virulence
- Metabolic adaptation to iron limitation upregulates virulence factors
- Metabolic flexibility enables survival during phagocytosis by immune cells
- Production of secreted hydrolytic enzymes regulated by nutrient availability
- Metabolic changes during host invasion support tissue penetration and dissemination
Metabolic Basis of Antifungal Resistance:
- Modifications in ergosterol biosynthesis pathway confer azole resistance
- Upregulation of drug efflux pumps requires energy from primary metabolism
- Metabolic dormancy in biofilm cells contributes to treatment failure
- Alternative metabolic pathways activated during drug exposure
- Metabolic adaptations can reduce drug uptake or enhance detoxification
- Stress responses triggered by antifungals involve metabolic reprogramming
- Metabolic plasticity contributes to development of resistance during treatment
The metabolic versatility of C. albicans is a key factor in its ability to colonize diverse host niches and transition between commensal and pathogenic states. Understanding the metabolic activities of this fungus provides insights into its ecological interactions within the human microbiome and may reveal new targets for therapeutic intervention. As research continues to elucidate the complex metabolic networks of C. albicans, novel approaches to disrupting pathogenic metabolism while preserving commensal relationships may emerge.
Clinical Relevance
Candida albicans has significant clinical relevance across multiple medical specialties due to its role in various infections and its complex interactions with the human host:
Diagnostic Challenges:
- Distinguishing between commensal colonization and pathogenic infection
- Limitations of traditional culture methods in detecting invasive candidiasis
- Need for rapid diagnostic techniques for early intervention in serious infections
- Biomarkers such as (1,3)-β-D-glucan and mannan antigens used for invasive disease
- Molecular techniques including PCR and DNA sequencing improving species identification
- Point-of-care testing being developed for rapid diagnosis in resource-limited settings
- Antifungal susceptibility testing increasingly important due to rising resistance
Oral Medicine and Dentistry:
- Oral candidiasis (thrush) common in denture wearers, infants, and immunocompromised patients
- Clinical presentations include pseudomembranous, erythematous, and hyperplastic forms
- Angular cheilitis affects corners of the mouth, often with bacterial co-infection
- Median rhomboid glossitis presents as a characteristic lesion on the tongue
- Denture stomatitis affects up to 65% of denture wearers
- Management includes improved oral hygiene, antifungal therapy, and addressing predisposing factors
- Recurrent oral candidiasis may indicate underlying immunodeficiency or HIV infection
Gynecology and Women's Health:
- Vulvovaginal candidiasis affects 75% of women at least once in their lifetime
- Recurrent vulvovaginal candidiasis (≥4 episodes/year) affects 5-8% of women
- Risk factors include pregnancy, diabetes, antibiotic use, and immunosuppression
- Symptoms include itching, burning, dyspareunia, and characteristic white discharge
- Treatment includes topical and oral azoles, with maintenance therapy for recurrent cases
- Emerging resistance to azole antifungals complicating management
- Potential role in adverse pregnancy outcomes being investigated
Gastroenterology:
- Esophageal candidiasis common in HIV/AIDS and other immunocompromised states
- Gastrointestinal colonization may serve as reservoir for invasive infection
- Potential association with inflammatory bowel disease and irritable bowel syndrome
- Emerging evidence for role in gut inflammation and barrier dysfunction
- Candida peritonitis can complicate peritoneal dialysis and abdominal surgery
- Management of GI candidiasis includes systemic antifungals and addressing underlying conditions
- Fecal microbiota transplantation being investigated for recurrent Candida overgrowth
Dermatology:
- Cutaneous candidiasis presents in intertriginous areas, nail folds, and diaper regions
- Intertrigo affects skin folds where moisture accumulates
- Paronychia involves inflammation of tissue surrounding nails
- Diaper dermatitis common in infants
- Chronic mucocutaneous candidiasis associated with specific immune defects
- Treatment includes topical antifungals, keeping affected areas dry, and addressing predisposing factors
- Differential diagnosis includes other fungal infections, bacterial infections, and dermatoses
Critical Care Medicine:
- Candidemia and invasive candidiasis significant causes of morbidity and mortality in ICU
- Risk factors include central venous catheters, parenteral nutrition, broad-spectrum antibiotics
- Mortality rates for candidemia approach 40% despite treatment
- Empiric antifungal therapy often initiated in high-risk patients
- Source control (e.g., catheter removal) crucial for successful treatment
- Echinocandins recommended as first-line therapy for invasive disease
- Prevention strategies include antifungal prophylaxis in selected high-risk populations
Infectious Disease Management:
- Increasing antifungal resistance complicating treatment
- Azole resistance mechanisms include target enzyme mutations and efflux pump overexpression
- Echinocandin resistance emerging, particularly with prolonged therapy
- Multidrug-resistant C. albicans strains reported globally
- Antifungal stewardship programs being implemented to preserve drug efficacy
- Combination therapy investigated for difficult-to-treat infections
- Novel antifungal classes in development to address resistance challenges
Special Populations:
- Neonates: Invasive candidiasis associated with prematurity and low birth weight
- Elderly: Higher risk of infection due to comorbidities and age-related immune changes
- HIV/AIDS: Oropharyngeal and esophageal candidiasis common opportunistic infections
- Transplant Recipients: High risk for invasive disease due to immunosuppression
- Cancer Patients: Neutropenia from chemotherapy increases susceptibility
- Diabetics: Elevated glucose levels promote Candida growth and impair immune function
- Surgical Patients: Risk of wound infections and invasive disease
The clinical relevance of C. albicans spans from common, easily treated superficial infections to life-threatening invasive disease. Management approaches must consider the specific clinical presentation, host factors, and potential for antifungal resistance. As our understanding of C. albicans pathogenesis continues to evolve, new diagnostic and therapeutic strategies are being developed to improve outcomes across the spectrum of Candida infections. The emergence of antifungal resistance presents a particular challenge that requires coordinated efforts in antifungal stewardship, development of novel antifungals, and alternative approaches to prevention and treatment.
Interactions with Other Microorganisms
Candida albicans engages in complex interactions with diverse microorganisms across various human body niches, influencing both microbial community dynamics and host health:
Bacterial-Fungal Antagonism:
- Lactobacillus species inhibit C. albicans through multiple mechanisms:
- Production of lactic acid creating unfavorable pH
- Secretion of hydrogen peroxide with antifungal activity
- Production of bacteriocins and biosurfactants
- Competition for adhesion sites and nutrients
- Pseudomonas aeruginosa produces quorum sensing molecules that inhibit hyphal formation
- Streptococcus mutans produces bacteriocins with anti-Candida activity
- Enterococcus faecalis secretes EntV, a bacteriocin that disrupts C. albicans biofilm formation
- Antagonistic interactions help maintain fungal homeostasis in healthy individuals
- Disruption of these interactions (e.g., through antibiotics) can lead to Candida overgrowth
- Lactobacillus species inhibit C. albicans through multiple mechanisms:
Bacterial-Fungal Synergism:
- Staphylococcus aureus forms mixed biofilms with C. albicans, enhancing virulence of both organisms
- C. albicans provides attachment sites for Streptococcus gordonii in oral biofilms
- Escherichia coli can enhance C. albicans adhesion to epithelial surfaces
- Bacteroides species may promote C. albicans colonization through cross-feeding relationships
- Synergistic interactions can increase resistance to antimicrobials and host defenses
- Co-infection with certain bacteria can lead to more severe disease manifestations
Metabolic Interactions:
- Cross-feeding relationships where metabolic byproducts of one organism serve as nutrients for another
- C. albicans can utilize short-chain fatty acids produced by anaerobic gut bacteria
- Some bacteria break down complex carbohydrates, making simpler sugars available to C. albicans
- C. albicans metabolism can alter local oxygen levels, creating niches for facultative anaerobes
- Competition for limited nutrients such as iron and zinc influences community composition
- Metabolic interactions contribute to spatial organization within polymicrobial communities
Biofilm Dynamics:
- Formation of complex, mixed-species biofilms on mucosal surfaces and medical devices
- Bacterial-fungal biofilms often exhibit enhanced structural integrity and resistance properties
- Extracellular matrix components from different species contribute to overall biofilm architecture
- Spatial organization within biofilms creates microenvironments with distinct properties
- Quorum sensing molecules mediate communication between species in biofilms
- Dispersal from biofilms can seed new sites of colonization or infection
Modulation of Virulence:
- Bacterial products can enhance or suppress expression of C. albicans virulence factors
- Peptidoglycan fragments released by bacteria during antibiotic treatment can promote C. albicans hyphal formation
- Bacterial lipopolysaccharide (LPS) can influence C. albicans morphogenesis and biofilm formation
- C. albicans secreted aspartyl proteases can degrade bacterial virulence factors
- Interspecies interactions can alter gene expression profiles in both fungi and bacteria
- Virulence modulation affects pathogenic potential and host immune responses
Influence on Host Responses:
- Mixed fungal-bacterial communities elicit different immune responses than single-species infections
- Bacterial-fungal interactions can enhance or suppress inflammatory responses
- Some bacterial species can protect against C. albicans infection by stimulating protective immunity
- C. albicans can influence bacterial recognition by host immune cells
- Polymicrobial interactions affect epithelial barrier function and mucosal immunity
- Host responses to mixed communities can differ from the sum of responses to individual species
Ecological Succession and Community Resilience:
- Temporal changes in microbial communities influence C. albicans abundance and behavior
- Early colonizers can create conditions that favor or inhibit subsequent C. albicans establishment
- C. albicans influences recolonization patterns following perturbations such as antibiotic treatment
- Stable communities with high diversity often suppress C. albicans overgrowth
- Resilience to perturbations depends on complex interaction networks among community members
- Understanding succession patterns may inform strategies to restore healthy communities
Clinical Implications of Polymicrobial Interactions:
- Mixed infections often more difficult to treat than single-species infections
- Bacterial-fungal interactions can influence antifungal and antibiotic efficacy
- Probiotic approaches targeting beneficial bacteria may help control C. albicans overgrowth
- Diagnostic challenges in distinguishing polymicrobial infections from single-species infections
- Treatment strategies increasingly considering the polymicrobial nature of many infections
- Potential for targeting interspecies interactions as novel therapeutic approach
The interactions between C. albicans and other microorganisms represent a complex ecological network that influences both microbial community dynamics and host health. These interactions range from antagonistic relationships that help maintain homeostasis to synergistic associations that can enhance pathogenicity. Understanding the mechanisms and consequences of these interactions provides insights into the ecological role of C. albicans in the human microbiome and may inform novel approaches to preventing and treating Candida infections. As research continues to elucidate the intricate relationships between fungi and bacteria in human-associated microbial communities, new strategies for manipulating these interactions to promote health may emerge.
Research Significance
Candida albicans holds substantial research significance across multiple scientific disciplines, with implications for basic biology, clinical medicine, and biotechnology:
Model Organism for Fungal Biology:
- Serves as a premier model for studying fungal cell biology and genetics
- Provides insights into eukaryotic cellular processes and stress responses
- Enables investigation of morphological plasticity and phenotypic switching
- Facilitates understanding of fungal-specific biological processes
- Offers opportunities to study eukaryotic pathogenesis mechanisms
- Advances in C. albicans research often applicable to other fungal species
- Genome sequencing and genetic manipulation tools continue to expand research capabilities
Host-Pathogen Interaction Studies:
- Exemplifies the transition from commensal to pathogen, informing broader concepts in microbial pathogenesis
- Illuminates mechanisms of immune recognition and evasion
- Provides a model for studying mucosal immunity and barrier function
- Reveals strategies pathogens use to adapt to diverse host environments
- Helps define the balance between tolerance and immunity to commensal organisms
- Contributes to understanding how microbes modulate host responses
- Informs development of immunotherapeutic approaches for fungal infections
Microbiome Research:
- Represents a key component of the human mycobiome (fungal microbiome)
- Provides insights into inter-kingdom interactions within microbial communities
- Helps elucidate factors governing microbial community assembly and stability
- Contributes to understanding dysbiosis and its role in disease
- Serves as a model for studying how fungi influence and are influenced by bacterial communities
- Advances methods for studying fungi in complex microbial ecosystems
- Informs approaches to manipulate microbiomes for health benefits
Antifungal Drug Discovery and Resistance:
- Critical for developing and testing novel antifungal compounds
- Provides insights into mechanisms of antifungal resistance
- Helps identify new drug targets through understanding of essential fungal processes
- Enables screening of compound libraries for antifungal activity
- Facilitates development of combination therapies and alternative approaches
- Informs antifungal stewardship strategies to preserve drug efficacy
- Serves as a model for studying evolution of drug resistance in microbial populations
Biofilm Research:
- Represents a leading model for studying fungal biofilm formation and regulation
- Provides insights into polymicrobial biofilm dynamics
- Helps elucidate mechanisms of biofilm-associated drug resistance
- Informs development of anti-biofilm strategies for medical devices
- Contributes to understanding environmental persistence of microorganisms
- Advances methods for biofilm visualization and analysis
- Bridges basic science and clinical applications in biofilm-related infections
Biotechnology Applications:
- Genetic and metabolic engineering for production of biopharmaceuticals
- Development of biosensors using C. albicans components
- Creation of fungal-based vaccine platforms
- Exploration of C. albicans enzymes for industrial applications
- Utilization of C. albicans cell wall components in immunomodulatory products
- Potential applications in bioremediation and environmental monitoring
- Development of diagnostic platforms for fungal detection
Clinical Research Advances:
- Drives development of improved diagnostic methods for invasive candidiasis
- Informs clinical guidelines for prevention and management of Candida infections
- Provides basis for personalized approaches to high-risk patients
- Contributes to understanding of fungal involvement in complex diseases
- Helps identify biomarkers for disease progression and treatment response
- Informs design of clinical trials for new antifungal agents
- Bridges laboratory findings to bedside applications
Future Research Directions:
- Integration of multi-omics approaches to understand C. albicans in complex environments
- Development of targeted approaches to disrupt pathogenicity while preserving commensal functions
- Exploration of the "mycobiome-gut-brain axis" and potential roles in neurological conditions
- Investigation of C. albicans interactions with the virome and parasitome
- Application of systems biology approaches to model C. albicans behavior in diverse contexts
- Development of immunotherapeutic strategies targeting fungal-specific pathways
- Exploration of the evolutionary history and ongoing evolution of C. albicans in human populations
The research significance of C. albicans extends from fundamental questions about fungal biology to practical applications in medicine and biotechnology. As a organism that exists at the intersection of commensalism and pathogenicity, C. albicans provides unique opportunities to study the factors that govern this transition and the complex interactions between microbes and their hosts. Continued research on this important fungal species promises to yield insights that will advance our understanding of microbial ecology, host-microbe interactions, and approaches to preventing and treating fungal diseases. The translational potential of C. albicans research is substantial, with implications for improving diagnosis, treatment, and prevention of fungal infections that affect millions of people worldwide.
