Overview
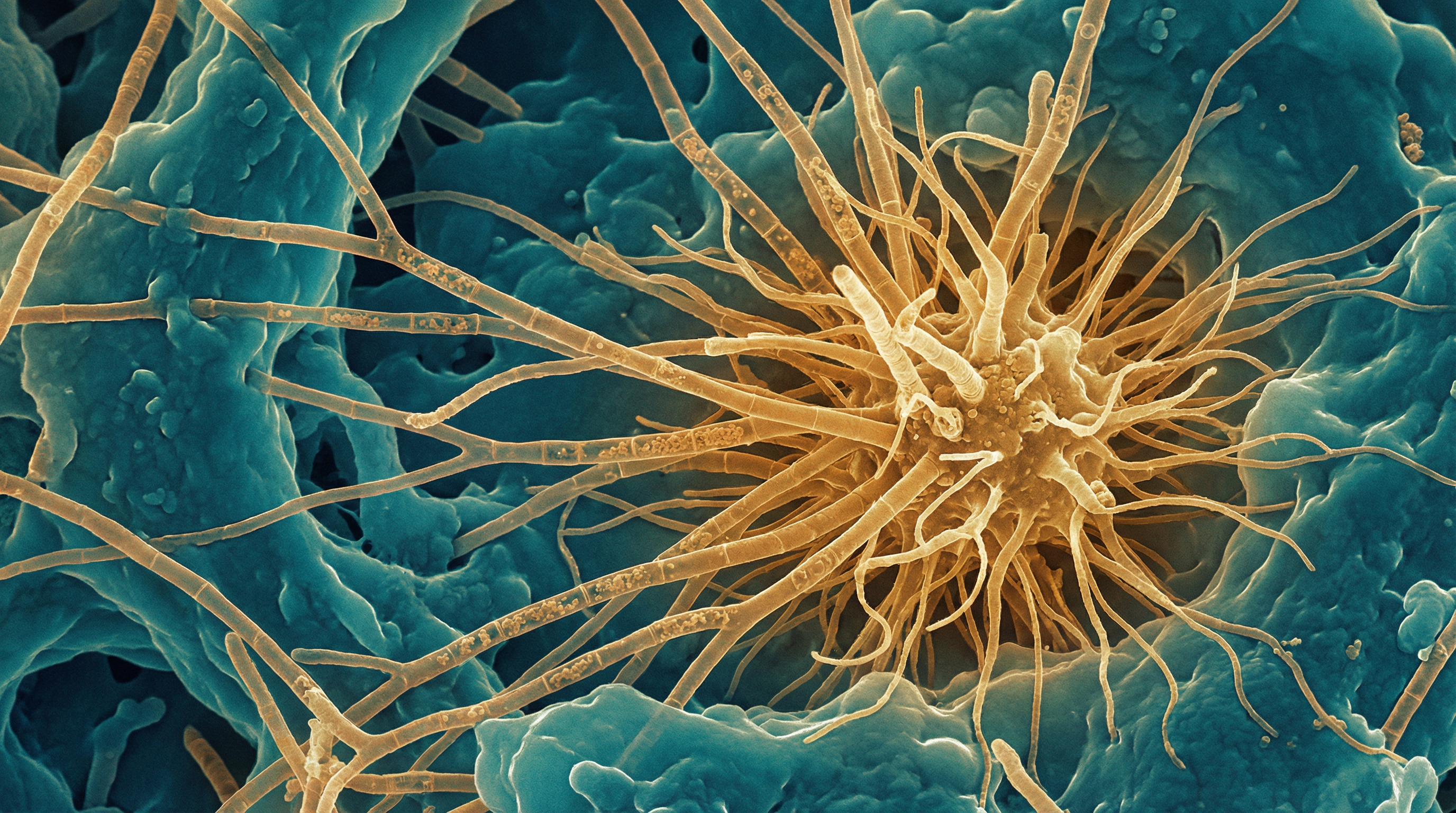
Actinomyces israelii is a Gram-positive, filamentous, anaerobic to microaerophilic bacterium that is a natural inhabitant of the human oral cavity, gastrointestinal tract, and urogenital tract. It is the most prevalent Actinomyces species isolated in human infections, responsible for approximately 70% of orocervicofacial actinomycosis cases.[1]
Key Characteristics
A. israelii is characterized by distinctive features:[2]
- Morphology: Branching, filamentous bacteria with a "molar tooth" colony appearance on agar
- Metabolism: Obligate or facultative anaerobe; slow-growing (5-14 days for colonies)
- Sulfur granules: Forms pathognomonic yellowish granules (40-400 μm) composed of tangled bacterial filaments
- Catalase-negative: Distinguishing biochemical characteristic
- Non-spore-forming, non-motile: Lacks flagella
Role in the Human Microbiome
As a commensal organism, A. israelii colonizes multiple body sites:[3]
Oral Cavity
- Dental plaque and biofilms (supragingival and subgingival)
- Gingival crevices and periodontal pockets
- Tonsillar crypts
- Tongue surface (associated with oral malodor)
Gastrointestinal Tract
- Part of the alimentary tract microbiota
- Can cause abdominal actinomycosis, particularly involving the ileocecal region
Urogenital Tract
- Normal inhabitant of female genital tract
- Colonization promoted by intrauterine device (IUD) use
Biofilm Formation and Bacterial Interactions
A. israelii plays a central role in oral biofilm ecology:[6]
Early Colonization
- Acts as a primary colonizer of tooth surfaces
- Provides attachment sites for secondary colonizers through coaggregation
Coaggregation Partners
- Streptococcus oralis and S. gordonii: Type 2 fimbriae recognize streptococcal receptor polysaccharides (RPS)
- Fusobacterium nucleatum: Acts as a bridging organism connecting early and late colonizers
- Prevotella loescheii: Serves as coaggregation bridge between A. israelii and other species
- Veillonella and Campylobacter gracilis: Significant coaggregation partners in root caries
IUD Biofilm Formation
In vitro studies demonstrate A. israelii can form spider-like colonies and porous biofilm structures on copper IUD surfaces, attaching via extracellular polymer production.[5]
Pathogenesis of Actinomycosis
Actinomycosis occurs when mucosal barriers are breached:[2]
Predisposing Factors
- Trauma (dental procedures, surgery, injury)
- Poor oral hygiene
- Foreign bodies (IUDs, dental implants)
- Immunosuppression
- Chronic inflammation
- Tissue devitalization
Disease Mechanism
- Bacteria gain access to deeper tissues through mucosal disruption
- Form dense masses of interlinked branched filaments that inhibit phagocytic clearance
- Produce proteolytic enzymes enabling tissue invasion
- Create abscesses with characteristic sulfur granules
- Spread contiguously across tissue planes (not lymphatically)
Clinical Forms
| Form | Frequency | Key Features |
|---|---|---|
| Cervicofacial | 50-65% | "Lumpy jaw"; mandible, cheeks, chin involvement |
| Thoracic | 15-30% | Following aspiration; pulmonary involvement |
| Abdominopelvic | 20-32% | IUD-associated; ileocecal predilection |
| CNS | 2-4% | Brain abscesses most common |
IUD-Associated Pelvic Actinomycosis
A well-documented association exists between IUD use and pelvic actinomycosis:[4]
- Colonization rate: 7% of IUD users have Actinomyces-like organisms on Pap smear (range 1.6-44%)
- Risk factors: Prolonged IUD use (>5 years); chronic endometrial inflammation
- Presentation: Pelvic abscesses, tubo-ovarian involvement, peritonitis
- Prevention: IUD replacement every 5 years recommended
Diagnosis
Gold Standards
- Culture: Slow-growing; requires anaerobic conditions; negative in 76% of cases
- Histopathology: Sulfur granules with Gram-positive branching filaments
- Molecular: 16S rRNA gene sequencing for species identification
Emerging Methods
- MALDI-TOF MS (limited accuracy for A. israelii)
- PCR-based detection
- ARDRA (Amplified 16S rDNA Restriction Analysis)
Treatment
A. israelii infections require prolonged antibiotic therapy:[2]
First-Line Treatment
- Intravenous: Penicillin G 12-24 million units/day for 2-6 weeks
- Oral maintenance: Penicillin V or amoxicillin for 6-12 months
Alternative Agents (Penicillin Allergy)
- Ceftriaxone
- Doxycycline
- Clindamycin
- Erythromycin
Key Considerations
- Intrinsically resistant to metronidazole
- Surgical debridement often required
- Combined medical-surgical cure rate >90%
- Recent evidence suggests some patients cured with <6 months therapy
References
Könönen E, Wade WG. Actinomyces and Related Organisms in Human Infections. Clinical Microbiology Reviews. 2015;28(2):419-442. doi:10.1128/CMR.00100-14
Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infection and Drug Resistance. 2014;7:183-197. doi:10.2147/IDR.S39601
Li J, Li Y, Zhou Y, et al. Actinomyces and Alimentary Tract Diseases: A Review of Its Biological Functions and Pathology. BioMed Research International. 2018;2018:3820215. doi:10.1155/2018/3820215
Gajdács M, Urban E. The Pathogenic Role of Actinomyces spp. and Related Organisms in Genitourinary Infections. Antibiotics. 2020;9(8):524. doi:10.3390/antibiotics9080524
Carrillo M, Valdez B, Vargas L, et al. In vitro Actinomyces israelii biofilm development on IUD copper surfaces. Contraception. 2010;81(3):261-264. doi:10.1016/j.contraception.2009.09.008
Kolenbrander PE, Andersen RN, Blehert DS, et al. Communication among Oral Bacteria. Microbiology and Molecular Biology Reviews. 2002;66(3):486-505. doi:10.1128/mmbr.66.3.486-505.2002
Bonnefond S, Catroux M, Melenotte C, et al. Clinical features of actinomycosis: A retrospective, multicenter study of 28 cases. Medicine. 2016;95(24):e3923. doi:10.1097/MD.0000000000003923
Heo SH, Shin SS, Kim JW, et al. Imaging of Actinomycosis in Various Organs: CT and MR Imaging Findings. RadioGraphics. 2014;34(1):19-33. doi:10.1148/rg.341135077
