Mycobacterium tuberculosis is the causative agent of tuberculosis (TB), one of the world's deadliest infectious diseases. While not typically considered part of the normal human microbiome, M. tuberculosis has significant interactions with the resident microbiota of the human body, particularly in the respiratory and gastrointestinal tracts. These interactions play crucial roles in disease pathogenesis, progression, and treatment outcomes.
Key Characteristics
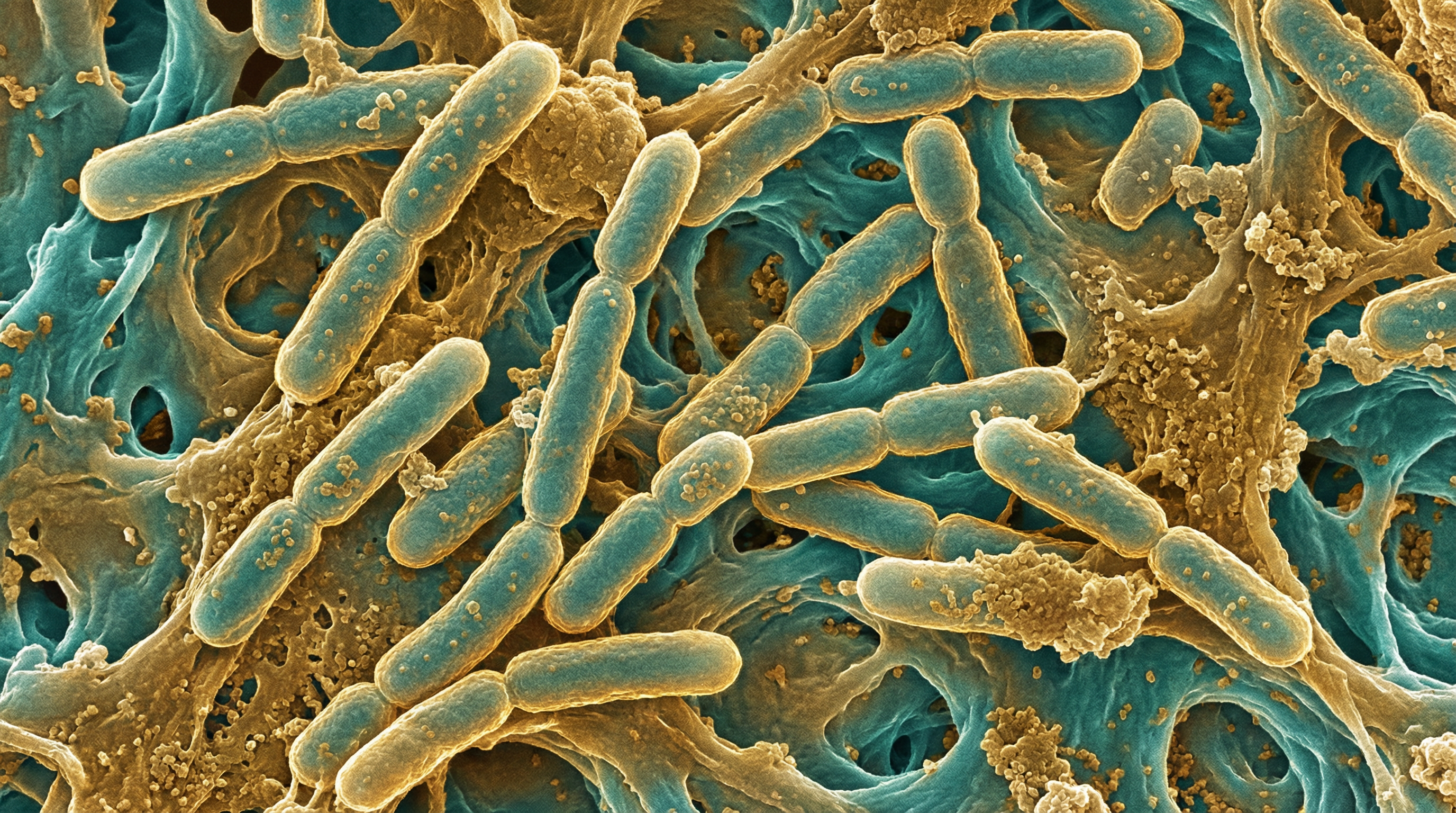
M. tuberculosis is a slow-growing, aerobic bacterium with a unique cell wall structure rich in mycolic acids, making it highly resistant to environmental stresses and many antibiotics. It is rod-shaped, non-motile, and does not form spores. Unlike many other bacteria in the human microbiome, M. tuberculosis is an obligate pathogen, meaning it does not typically exist as a commensal organism but rather causes disease when present in the human body.
The bacterium is transmitted through airborne droplets when individuals with active TB cough, sneeze, or speak. Once inhaled, M. tuberculosis primarily infects the lungs but can disseminate to other organs, causing extrapulmonary TB.
Interaction with the Human Microbiome
M. tuberculosis has complex interactions with the resident microbiota of both the respiratory and gastrointestinal tracts:
Lung Microbiome Interactions
- M. tuberculosis infection alters the composition and diversity of the lung microbiome, often leading to dysbiosis
- Studies have shown reduced microbial diversity in the respiratory tract of TB patients
- Certain lung microbiota may compete with M. tuberculosis for resources, potentially limiting its growth
- The lung microbiome may influence the formation and maintenance of granulomas, the hallmark of TB infection
- Changes in the lung microbiome composition may affect local immune responses, influencing TB disease progression
Gut Microbiome Interactions
- TB infection and disease are associated with altered gut microbiome composition and reduced diversity
- The gut-lung axis plays a significant role in immune responses to M. tuberculosis
- Gut microbiota produce metabolites that can modulate systemic immunity, potentially affecting TB outcomes
- Anti-TB medications can further disrupt the gut microbiome, potentially leading to adverse effects and treatment complications
- Specific gut bacteria may influence the metabolism of TB drugs, affecting their efficacy and toxicity
Impact on Human Health
M. tuberculosis is responsible for tuberculosis, which remains one of the top 10 causes of death worldwide. The bacterium can cause:
- Pulmonary TB, affecting the lungs and characterized by chronic cough, blood-tinged sputum, fever, night sweats, and weight loss
- Extrapulmonary TB, affecting organs outside the lungs, including the lymph nodes, bones, joints, urinary tract, and central nervous system
- Latent TB infection, where individuals are infected but do not have active disease and are not contagious
The interaction between M. tuberculosis and the human microbiome has several health implications:
- Dysbiosis caused by M. tuberculosis infection may contribute to disease progression and severity
- Changes in the microbiome may influence the effectiveness of TB treatments
- The microbiome may play a role in determining whether TB infection remains latent or progresses to active disease
- Microbiome composition may affect susceptibility to TB infection and reinfection
Immune System Interactions
M. tuberculosis has evolved sophisticated mechanisms to evade and manipulate the human immune system:
- It can survive and replicate within macrophages, the very cells meant to destroy it
- The bacterium induces the formation of granulomas, which both contain the infection and provide a niche for bacterial persistence
- M. tuberculosis modulates the balance between different T cell responses (Th1, Th2, Th17, and Treg)
- The microbiome influences these immune responses, potentially affecting TB outcomes
- Gut microbiota can enhance or suppress specific immune pathways relevant to TB control
Potential Therapeutic Approaches
Understanding the interactions between M. tuberculosis and the human microbiome opens new avenues for TB prevention and treatment:
- Microbiome-targeted interventions, such as probiotics or prebiotics, may help enhance immune responses against M. tuberculosis
- Fecal microbiota transplantation has been proposed as a potential adjunctive therapy for drug-resistant TB
- Specific commensal bacteria or their metabolites might be developed as novel anti-TB agents
- Preserving or restoring a healthy microbiome during TB treatment may improve outcomes and reduce adverse effects
- Microbiome profiles might serve as biomarkers for TB risk, progression, or treatment response
Research Challenges and Future Directions
Despite growing interest in the role of the microbiome in TB, several challenges remain:
- Establishing causal relationships between microbiome changes and TB outcomes
- Determining whether microbiome alterations are a cause or consequence of TB disease
- Identifying specific microbiota or microbial products that could be targeted therapeutically
- Developing practical microbiome-based interventions suitable for resource-limited settings where TB is most prevalent
- Understanding how host genetics, environment, and diet influence microbiome-TB interactions
Future research directions include longitudinal studies of microbiome changes during TB infection and treatment, mechanistic studies of how specific microbiota influence TB immunity, and clinical trials of microbiome-targeted interventions as adjuncts to standard TB therapy.
