Psoriasis
Discover the crucial connection between your microbiome and psoriasis, and learn evidence-based approaches for managing symptoms through gut and skin health.
Common Symptoms
Microbiome Imbalances
Research has identified the following microbiome patterns commonly associated with this condition:
- Reduced gut microbial diversity
- Decreased Faecalibacterium prausnitzii
- Increased Escherichia coli
- Altered skin microbiome
Understanding Psoriasis
Psoriasis is a chronic, immune-mediated inflammatory skin condition characterized by red, raised, scaly patches that typically appear on the knees, elbows, scalp, and trunk. Unlike eczema, which can be triggered by external irritants, psoriasis stems from internal immune dysregulation that accelerates skin cell turnover, causing cells to build up rapidly on the skin's surface.
The condition affects approximately 2-3% of the global population and can significantly impact quality of life through physical discomfort, visible symptoms, and associated conditions like psoriatic arthritis. Psoriasis is not merely a skin disorder but a systemic inflammatory condition with links to metabolic syndrome, cardiovascular disease, and inflammatory bowel disease.
While traditionally viewed primarily as an immune-mediated condition with genetic components, mounting evidence reveals that both the skin and gut microbiomes play crucial roles in psoriasis development, progression, and treatment response.
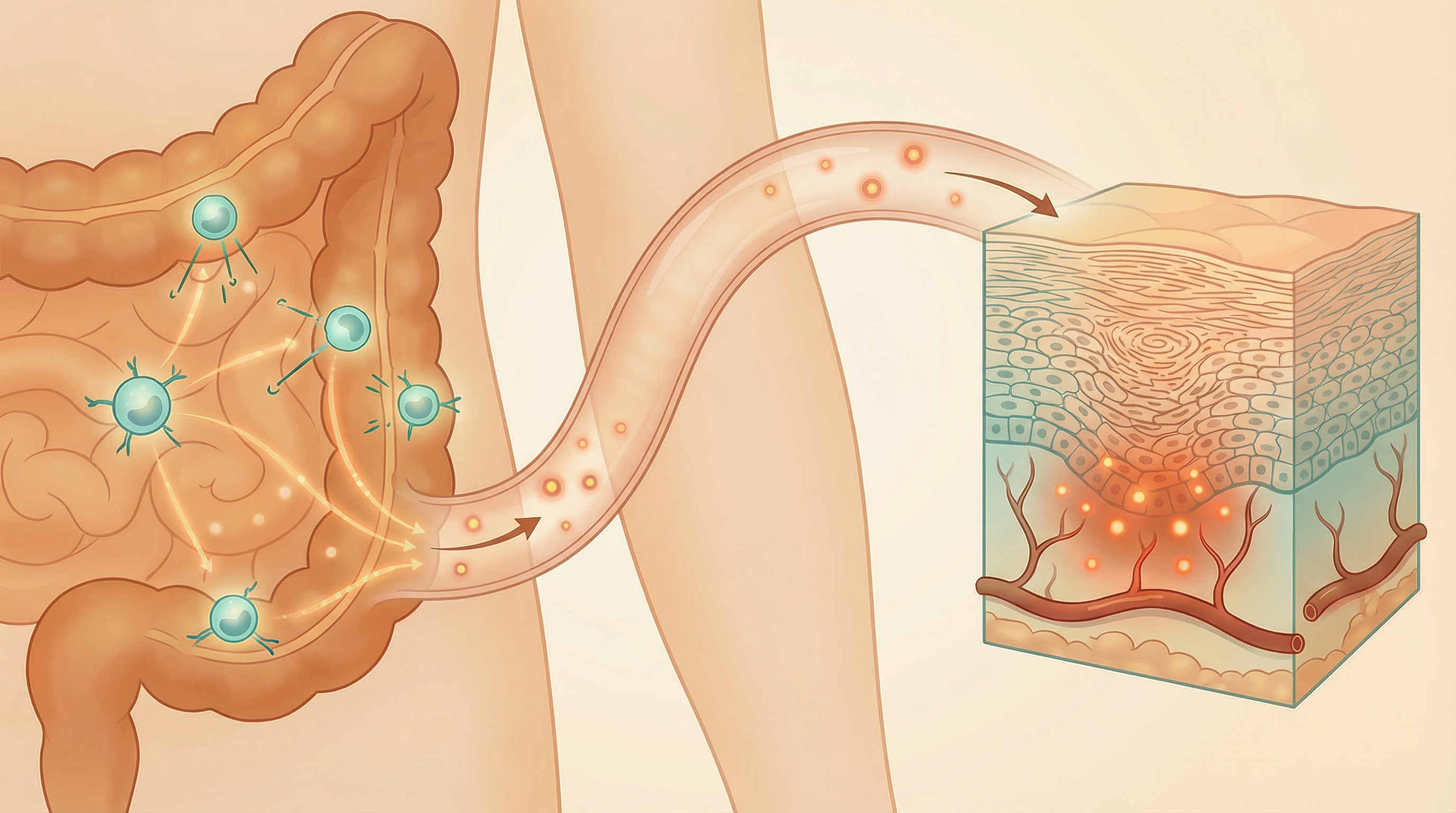
The Gut-Skin Axis in Psoriasis
Research has established a bidirectional communication pathway between the gut and skin—often called the "gut-skin axis." Psoriasis is characterized by gut dysbiosis and reduced microbial diversity, with decreased beneficial species like Faecalibacterium prausnitzii and increased proinflammatory species like Prevotella copri, which exacerbates skin inflammation through the gut-skin axis.[1] This connection helps explain why gut microbiome imbalances can manifest as skin inflammation and why addressing gut health can improve skin conditions like psoriasis.
Intestinal Permeability
Psoriasis patients often show increased intestinal permeability ("leaky gut"), allowing bacterial components like lipopolysaccharide (LPS) to enter circulation and trigger systemic inflammation that can manifest in the skin. A significant association exists between psoriasis and gut microbial alterations, characterized by lower relative abundance of Bacteroidetes and higher abundance of Firmicutes compared to healthy individuals.[2]
Immune Activation
The gut microbiome influences the balance of pro-inflammatory (Th1/Th17) and anti-inflammatory (Treg) immune cells. Dysbiosis can promote the Th17-dominant immune profile characteristic of psoriasis.
Microbial Metabolites
Short-chain fatty acids (SCFAs) and other metabolites produced by gut bacteria have anti-inflammatory effects. Reduced SCFA production is observed in many psoriasis patients, potentially contributing to inflammation.
Shared Inflammatory Pathways
The IL-23/IL-17 axis central to psoriasis pathogenesis is also influenced by gut microbiota, explaining the frequent co-occurrence of psoriasis and inflammatory bowel disease.
Psoriasis patients exhibit a significantly disturbed gut microbiota profile characterized by inverted ratios of Firmicutes and Bacteroidetes, with composition changes that correlate with disease severity and inflammatory markers.[3] Psoriasis is strongly associated with gut microbiota dysbiosis, and modulating the microbiome through dietary changes or probiotic and prebiotic supplementation represents a promising novel therapeutic and preventive approach.[4]
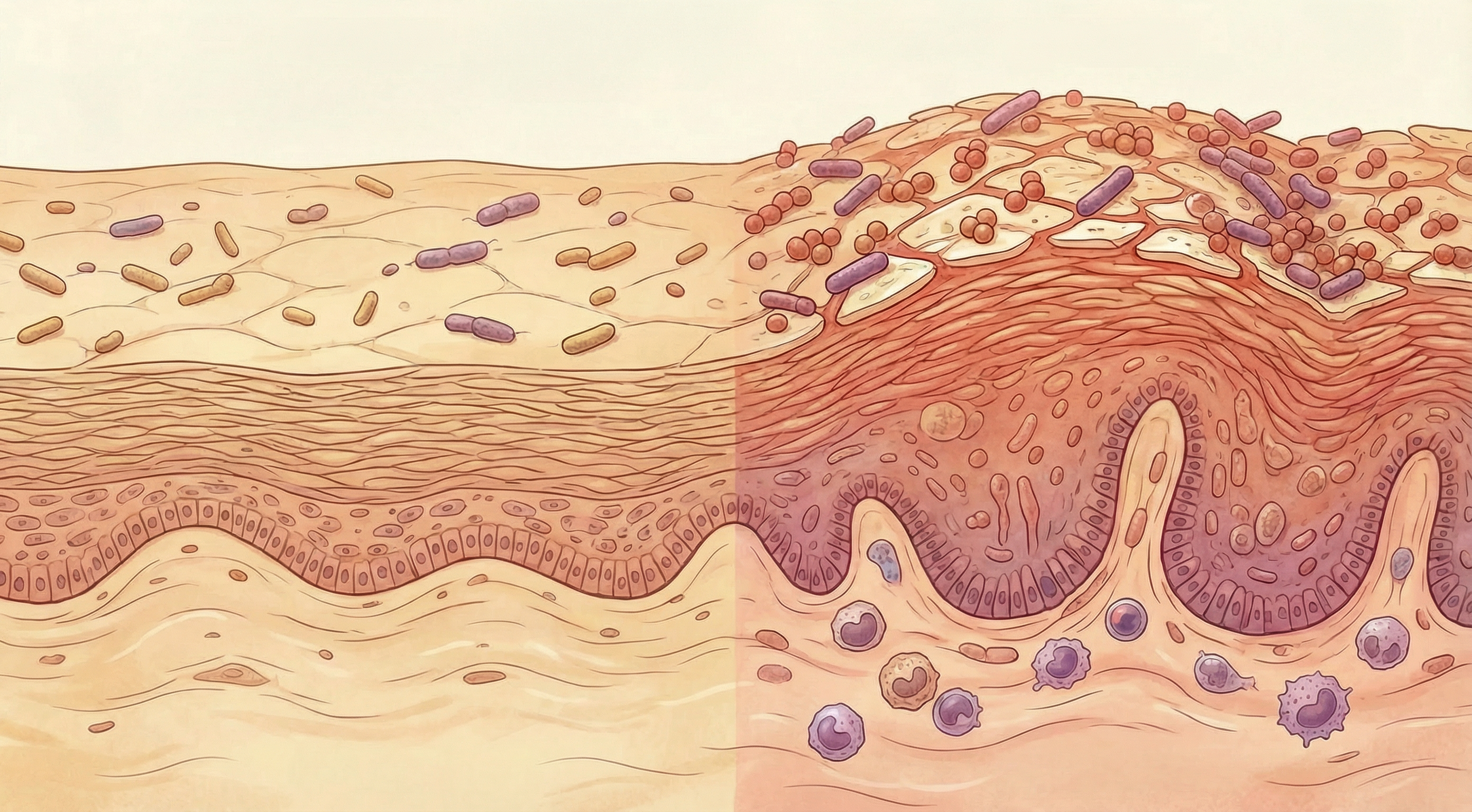
The Skin Microbiome in Psoriasis
The skin microbiome—the collection of microorganisms living on the skin surface—is significantly altered in psoriasis lesions compared to healthy skin:
Staphylococcus aureus
Impact: Potentially harmful, often increased in psoriatic lesions Function: Can trigger inflammatory responses and exacerbate skin barrier dysfunction
Cutibacterium acnes
Impact: Typically reduced in psoriatic lesions Function: Produces short-chain fatty acids that maintain skin pH and barrier function
Streptococcus species
Impact: Potentially harmful, associated with guttate psoriasis Function: Streptococcal throat infections can trigger guttate psoriasis through molecular mimicry
Malassezia species
Impact: Mixed; altered abundance in psoriasis Function: Can trigger immune responses in susceptible individuals, particularly in scalp psoriasis
Unlike eczema, where Staphylococcus aureus dominance is a consistent finding, the skin microbiome changes in psoriasis are more variable. However, research consistently shows reduced microbial diversity and altered community structure in psoriatic lesions compared to healthy skin from the same individuals.
Interestingly, the microbiome of uninvolved skin (areas without visible psoriasis) in psoriasis patients also differs from healthy controls, suggesting that microbiome alterations may precede visible lesions and could potentially serve as early biomarkers.
Microbiome-Based Approaches for Psoriasis Management
Emerging research supports several microbiome-focused strategies for managing psoriasis symptoms:
Anti-Inflammatory Diet
Mediterranean and plant-based diets rich in omega-3 fatty acids, polyphenols, and fiber can promote beneficial gut bacteria and reduce systemic inflammation. Clinical studies show these dietary patterns may reduce psoriasis severity and improve treatment response. Evidence Level: Moderate
Probiotics
Specific probiotic strains, particularly Lactobacillus and Bifidobacterium species, may help reduce inflammation and improve psoriasis symptoms. A 2025 meta-analysis found that gut microbiota modulation via probiotics significantly reduces psoriasis severity and systemic inflammation by enhancing the gut-skin axis, notably outperforming traditional systemic pharmacological therapies and synbiotics.[5] Effects appear to be strain-specific and may vary between individuals. Evidence Level: Moderate
Prebiotics
Dietary fibers that selectively feed beneficial bacteria can improve gut microbiome composition and reduce inflammation. Inulin, fructo-oligosaccharides (FOS), and resistant starch have shown particular promise for modulating immune responses relevant to psoriasis. Evidence Level: Preliminary
Vitamin D
Beyond its direct effects on skin cells, vitamin D influences both gut and skin microbiome composition. Adequate vitamin D levels are associated with greater microbial diversity and reduced inflammation in psoriasis patients. Evidence Level: Moderate
These microbiome-based approaches are most effective when integrated with conventional psoriasis management strategies, including topical treatments, phototherapy, and systemic medications when necessary. The optimal approach likely involves addressing both skin and gut microbiomes simultaneously.

The Psoriasis-Arthritis-IBD Connection
Psoriasis shares genetic risk factors, inflammatory pathways, and microbiome alterations with psoriatic arthritis and inflammatory bowel disease, forming what some researchers call the "psoriatic disease spectrum."
Shared Features Across the Psoriatic Disease Spectrum
- Genetic Factors: IL23R, IL12B, and CARD9 variants are associated with psoriasis, psoriatic arthritis, and IBD
- Immune Pathways: The IL-23/IL-17 axis is central to all three conditions
- Microbiome Changes: Reduced diversity and similar taxonomic shifts are observed in the gut microbiome
- Treatment Response: Similar biologic therapies targeting TNF-α and IL-23/IL-17 are effective across these conditions
This connection explains why approximately 30% of psoriasis patients develop psoriatic arthritis and why psoriasis patients have a 3-7 fold increased risk of inflammatory bowel disease. It also suggests that addressing the microbiome could potentially benefit multiple aspects of psoriatic disease simultaneously.
Future Directions in Microbiome-Based Psoriasis Treatment
The field is rapidly evolving, with several promising approaches on the horizon:
Precision Microbiome Modulation
Research is increasingly focusing on identifying specific microbiome signatures that might predict response to different treatments, potentially enabling more personalized approaches to psoriasis management.
Novel Interventions
Beyond traditional probiotics, researchers are exploring next-generation approaches including:
- Engineered live biotherapeutics designed to target specific aspects of psoriasis pathophysiology
- Postbiotics derived from beneficial microorganisms that may have direct anti-inflammatory effects
- Fecal microbiota transplantation (FMT) which has shown preliminary promise in small studies
Integrated Approaches
The most promising future direction involves integrating microbiome-based therapies with conventional treatments, potentially allowing for lower doses of immunosuppressive medications and reduced side effects.
The integration of microbiome science into psoriasis management represents a paradigm shift toward addressing underlying biological mechanisms rather than just managing symptoms. While much research remains to be done, the evidence to date suggests that nurturing healthy skin and gut microbiomes may be an important component of comprehensive psoriasis care.
Research Summary
Research has established a bidirectional communication pathway between the gut and skin—often called the 'gut-skin axis.' This connection helps explain why gut microbiome imbalances can manifest as skin inflammation and why addressing gut health can improve skin conditions like psoriasis.
References
- Annunziata G, Verde L, Zink A, et al.. The Gut–Skin Axis in Psoriasis: A Narrative Review. Current Nutrition Reports. 2025;14(1):41. doi:10.1007/s13668-024-00587-y ↩
- Sikora M, Chrabąszcz M, Maciejewska K, et al.. Intestinal Microbiome in Psoriasis: A Systematic Review. Pathogens. 2020;9(6):463. doi:10.3390/pathogens9060463 ↩
- Huang L, Gao R, Yu N, et al.. Dysbiosis of gut microbiota was closely associated with psoriasis. Science China Life Sciences. 2019;62(6):807-815. doi:10.1007/s11427-018-9376-6 ↩
- Buhaș MC, Gavrilaș LI, Candrea R, et al.. Gut Microbiota in Psoriasis. Nutrients. 2022;14(14):2970. doi:10.3390/nu14142970 ↩
- Amalia N, Wicaksono D, Wiyarta E, et al.. The gut-skin axis in psoriasis: Evidence-based insights from a meta-analysis on probiotics-synbiotics-mediated microbiota interventions. Medicine in Microecology. 2025;25:100126. doi:10.1016/j.medmic.2025.100126 ↩
