Overview
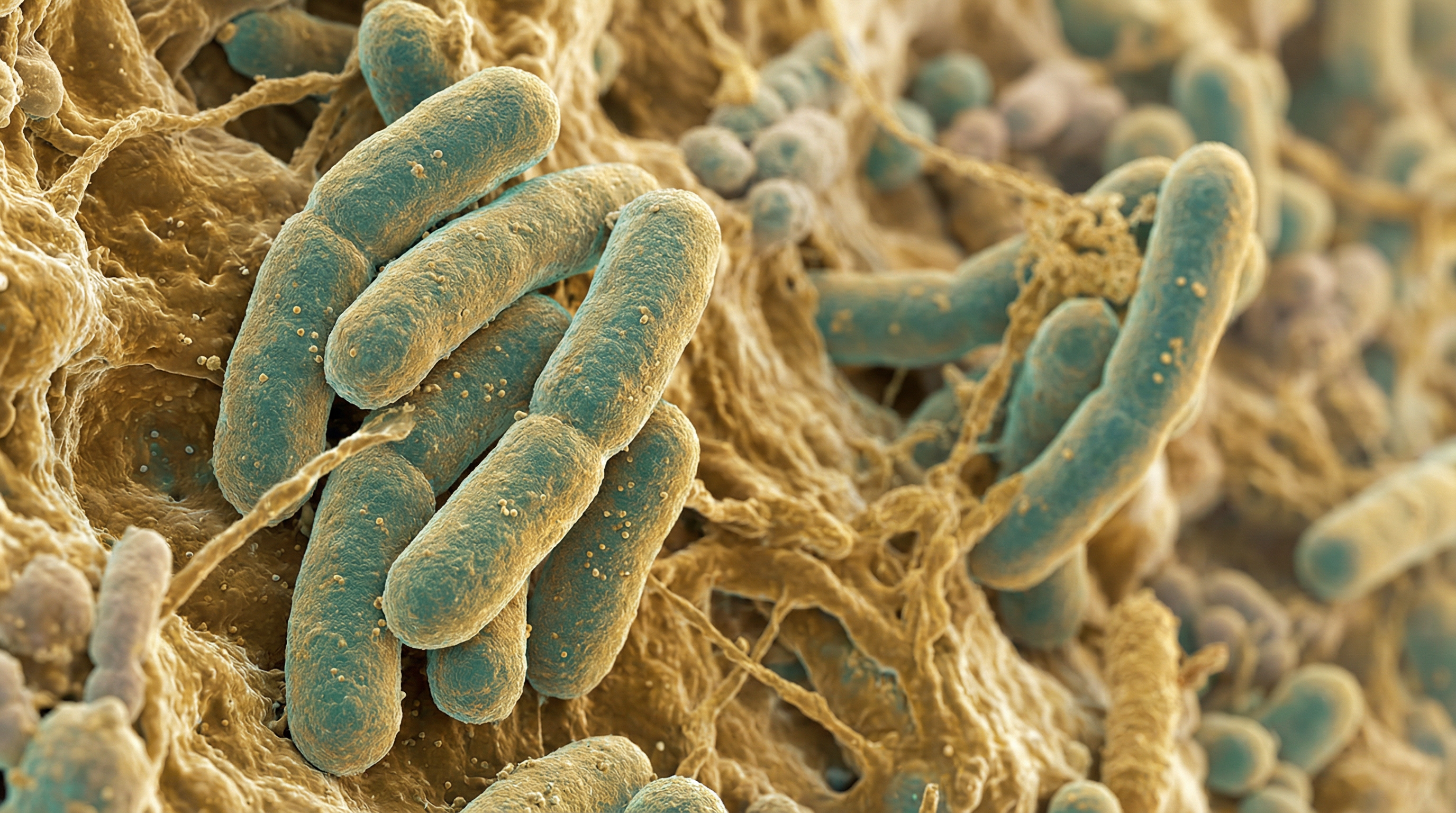
Faecalibacterium prausnitzii is a Gram-positive, strictly anaerobic, non-spore-forming commensal bacterium that constitutes 5-15% of total gut bacteria in healthy adults, making it one of the most abundant species in the human intestinal microbiota. It has emerged as a cornerstone of gut health due to its potent anti-inflammatory properties and role as a major butyrate producer.[1]
Butyrate Production and Gut Health
F. prausnitzii is a major producer of butyrate, the primary energy source for colonocytes. Butyrate production is responsible for many of the species' beneficial effects:[4]
- Dact3 pathway: Butyrate upregulates Dact3 gene expression, which negatively regulates Wnt/JNK signaling and blocks IL-8 production
- SCFA ratios: Shifts SCFA profile toward healthy 3:1:1 ratio (acetate:propionate:butyrate)
- Gut barrier: Enhances tight junction integrity and mucus layer maintenance
- HDAC modulation: Influences epigenetic regulation through histone deacetylase inhibition
Anti-Inflammatory Mechanisms
Microbial Anti-inflammatory Molecule (MAM)
A landmark 2016 study identified a 15 kDa protein named MAM (Microbial Anti-inflammatory Molecule) that inhibits NF-κB pathway activation in intestinal epithelial cells in a dose-dependent manner.[2]
Immune Modulation
F. prausnitzii exerts comprehensive immunomodulatory effects:[1]
- NF-κB inhibition: Blocks NF-κB activation and IL-8 production through secreted metabolites
- Cytokine profile: Stimulates high IL-10 secretion and low IL-12/IFN-γ in PBMCs
- Th17 suppression: Inhibits IL-23/Th17/IL-17 pathway
- Treg induction: Skews dendritic cells to prime IL-10-secreting Tr1-like regulatory T cells
- TLR2/6 signaling: Activates TLR2/6 and JNK pathway for immune tolerance
Protective Metabolites
Beyond MAM, F. prausnitzii produces other protective compounds:
- Salicylic acid: Blocks IL-8 production
- Shikimic acid: Associated with anti-inflammatory effects
- α-ketoglutarate: Modulates inflammatory pathways
IBD Association
Clinical Evidence
F. prausnitzii abundance is consistently reduced in inflammatory bowel disease:[5]
- Crohn's disease: Most pronounced reduction, especially with ileal involvement
- Ulcerative colitis: Significant reduction versus healthy controls
- Predictive value: Low ileal levels at surgery predict higher risk of endoscopic CD recurrence at 6 months
- Disease activity: Lower counts associated with increased disease activity and higher relapse rates
- Remission duration: Lower levels predict shorter clinical remission (<12 months)
DP8α Tregs
A 2022 study discovered that CD4+CD8α+ regulatory T cells (DP8α Tregs) specific to F. prausnitzii protect against intestinal inflammation. Low DP8α cell frequencies in IBD patients are associated with disease activity, flares, and elevated CRP.
Biomarker Potential
F. prausnitzii serves as a biomarker for multiple conditions:[5]
| Condition | Association |
|---|---|
| Crohn's disease | Significantly reduced; predicts recurrence |
| Ulcerative colitis | Reduced vs healthy controls |
| Type 2 diabetes | Significantly depleted |
| Chronic kidney disease | Depleted in Western and Eastern populations |
| Cognitive decline/MCI | Correlates with cognitive scores |
| Obesity/metabolic liver disease | Reduced; inversely correlated with hepatic fat |
Oxygen Sensitivity and Cultivation Challenges
F. prausnitzii is extremely oxygen-sensitive (EOS), creating significant challenges for therapeutic development:
Traditional Limitations
- Cannot survive standard probiotic manufacturing processes
- Dies rapidly upon exposure to ambient air
- Requires strict anaerobic conditions for cultivation
Breakthrough Solutions[3]
A 2023 Nature study achieved major advances:
- Symbiotic co-culture: Desulfovibrio piger provides acetate and acts as electron sink to promote growth
- Oxygen adaptation: Stepwise adaptation technology creates oxygen-tolerant strains
- Industrial production: Enables stable probiotic capsule manufacturing
- Clinical validation: Human trial (50 participants) showed safety, tolerability, and increased strain abundance
Protective Formulations
- Cysteine + riboflavin + inulin: Keeps bacterium alive at ambient air for 24 hours
- Corn starch + wheat bran granules: Achieves 60% viability
Clinical Trials and Therapeutic Applications
Type 2 Diabetes
F. prausnitzii supplementation in diabetic mice decreased fasting blood glucose, improved insulin resistance and glucose intolerance, and ameliorated hepatic steatosis by inhibiting lipogenic enzymes.
Cancer Immunotherapy
High baseline F. prausnitzii in NSCLC and melanoma patients is associated with better ICI response and overall survival. Strain EXL01 restores anti-tumor responses and reduces immunotherapy-induced colitis while enhancing T cell activation.
Chronic Kidney Disease[6]
Supplementation reduces renal dysfunction, inflammation, and fibrosis in mouse models. Effects are mediated by butyrate through renal GPR-43 receptor, with improvements in serum uremic toxins and intestinal barrier integrity.
Obesity and Metabolic Health
Oral administration reduced hepatic fat content, increased fatty acid oxidation, and decreased inflammation in visceral and subcutaneous adipose tissues, with improved insulin sensitivity.
Dietary Modulation
Research shows diet can influence F. prausnitzii levels:
- Kiwifruit: Supplementation increased abundance from 3.4% to 7.0% in constipated individuals
- Mediterranean diet: Fiber-rich diet boosts diversity in obese individuals
- Flavin/riboflavin-rich foods: May support survival at oxic-anoxic interfaces
References
Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proceedings of the National Academy of Sciences. 2008;105(43):16731-16736. doi:10.1073/pnas.0804812105
Quévrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut. 2016;65(3):415-425. doi:10.1136/gutjnl-2014-307649
Khan MT, Dwibedi C, Sundh D, et al. Synergy and oxygen adaptation for development of next-generation probiotics. Nature. 2023;620(7973):381-385. doi:10.1038/s41586-023-06378-w
Lenoir M, Martín R, Torres-Maravilla E, et al. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes. 2020;12(1):1826748. doi:10.1080/19490976.2020.1826748
Lopez-Siles M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M. Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. The ISME Journal. 2017;11(4):841-852. doi:10.1038/ismej.2016.176
Li HB, Xu ML, Xu XD, et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circulation Research. 2022;131(9):e120-e134. doi:10.1161/CIRCRESAHA.122.320184
Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis. Gastroenterology Research and Practice. 2014;2014:872725. doi:10.1155/2014/872725
Gao Y, Xu P, Sun D, et al. Faecalibacterium prausnitzii Abrogates Intestinal Toxicity and Promotes Tumor Immunity to Increase the Efficacy of Dual CTLA4 and PD-1 Checkpoint Blockade. Cancer Research. 2023;83(22):3710-3725. doi:10.1158/0008-5472.CAN-23-0605
