Clostridium butyricum
Overview
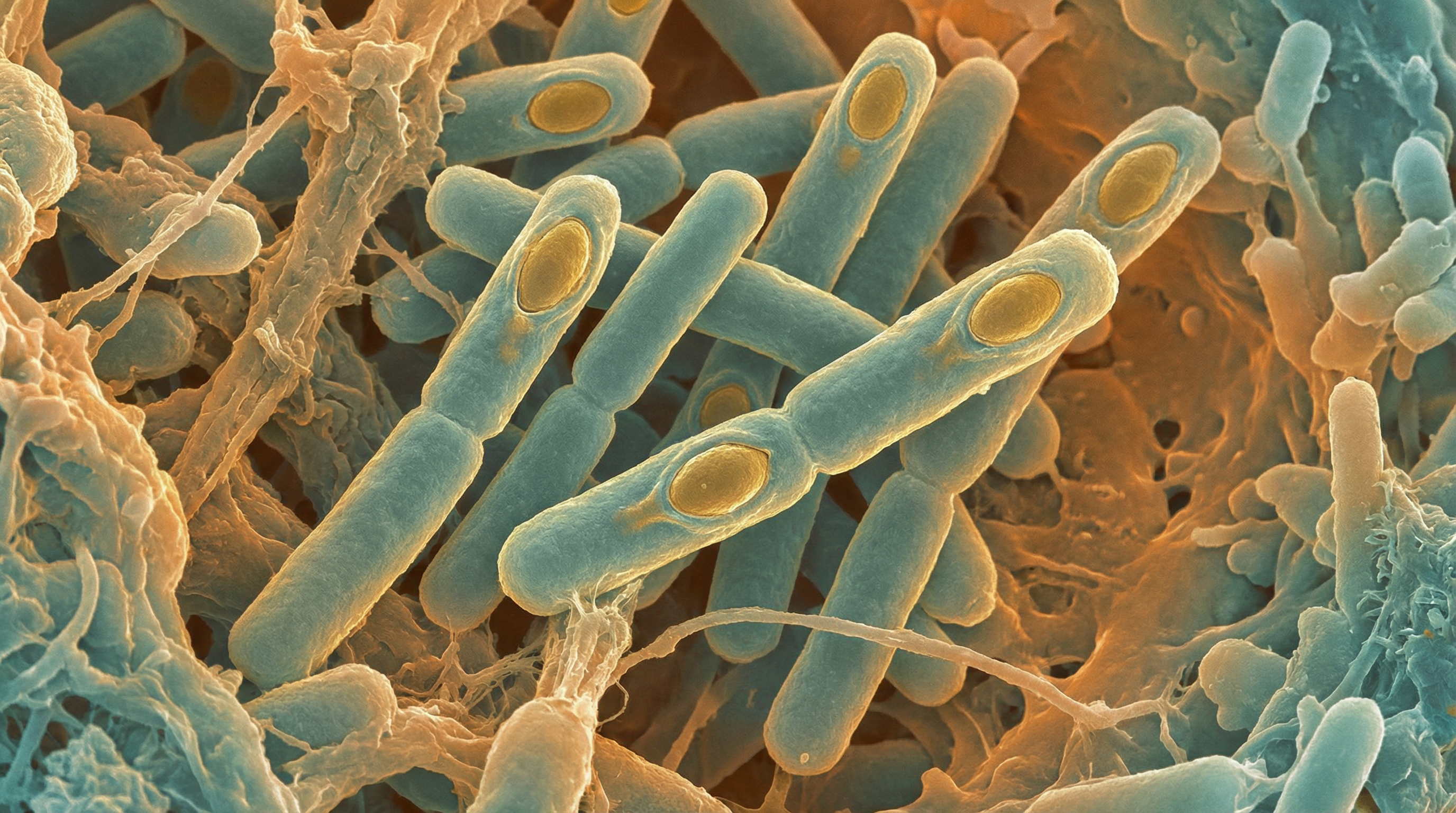
Clostridium butyricum is a Gram-positive, obligate anaerobic, spore-forming bacillus named for its capacity to produce high amounts of butyric acid. It is a common human and animal gut commensal bacterium that is also frequently found in soil, vegetables, and fermented foods like sour milk. C. butyricum has gained significant attention as a probiotic due to its beneficial effects on gut health, immune function, and protection against pathogens. It possesses remarkable characteristics including resistance to low pH, high temperatures, and multiple antibiotics, making it more resilient than many other probiotics.
Characteristics
C. butyricum is characterized by the following features:
- Gram-positive, rod-shaped bacterium
- Obligate anaerobe (grows in environments lacking oxygen)
- Spore-forming, which contributes to its environmental resilience
- Produces large amounts of gas in medium containing fermentable carbohydrates
- Strong acid and alkali resistance
- High temperature resistance
- Resistance to most antibiotics
- Capable of fermenting carbohydrates to produce short-chain fatty acids, especially butyric acid
- Possesses hydrophobicity and the capacity to degrade starch
- Gamma-hemolytic (non-hemolytic) on blood agar
Role in Human Microbiome
C. butyricum exists in a mutualistic relationship with the human host, primarily inhabiting the gastrointestinal tract. While not among the most abundant species in the gut microbiome, it plays several crucial roles:
Butyrate Production: C. butyricum is a major producer of butyrate, a short-chain fatty acid that serves as the primary energy source for colonocytes (intestinal epithelial cells).
Microbiome Modulation: It helps change the gut microbiome's composition by increasing the numbers of beneficial microbes such as Lactobacillus and Bifidobacterium, which aid digestion and protect against pathogens.
Gut Barrier Function: C. butyricum strengthens the intestinal barrier, maintaining the tolerance of gut microbiota while preventing the translocation of harmful bacteria.
Restoration of Gut Microflora: It helps restore the gut microbiota after disruption, such as following antibiotic treatment.
Nutrient Breakdown: It supports digestive health by helping to break down and absorb nutrients from complex foods such as whole grains and vegetables.
Health Implications
C. butyricum has numerous health implications, primarily beneficial:
Beneficial Effects:
Gastrointestinal Health:
- Treatment of antibiotic-associated diarrhea
- Prevention and treatment of inflammatory bowel diseases
- Improvement of intestinal structure and function
- Reduction of intestinal inflammation
Immune System Support:
- Enhancement of host immune function
- Promotion of a robust immune system by improving gut barrier function
- Prevention of harmful pathogens from colonizing the gut
Disease Prevention:
- Reduced risk of colorectal cancer
- Protection against bacterial infections, particularly from pathogens like Vibrio parahaemolyticus
- Potential protection against depression and other mental health conditions
Growth and Development:
- Improved growth performance in various organisms
- Enhanced nutrient absorption and utilization
Adverse Effects:
While C. butyricum is generally considered safe and beneficial, there have been rare reports of:
- C. butyricum bacteremia (bacteria in the bloodstream) associated with probiotic use in immunocompromised individuals
- Potential pathogenicity in certain contexts, particularly in vulnerable populations
Metabolic Activities
C. butyricum exhibits versatile metabolic capabilities that contribute to its ecological success and probiotic properties:
Carbohydrate Fermentation:
- Ferments various carbohydrates, particularly dietary fibers from whole grains, fruits, and vegetables
- Produces short-chain fatty acids (SCFAs) as end products of fermentation
Butyrate Production:
- Specialized in producing high amounts of butyric acid (butyrate)
- Butyrate serves as an energy source for intestinal epithelial cells and has anti-inflammatory properties
Gas Production:
- Generates hydrogen and carbon dioxide during fermentation
- This gas production is characteristic of its growth in media containing fermentable carbohydrates
Starch Degradation:
- Possesses amylolytic activity, enabling it to break down complex starches
- This ability contributes to its role in nutrient breakdown and absorption
Adaptation to Anaerobic Environments:
- Thrives in oxygen-free environments of the intestinal tract
- Metabolic pathways optimized for anaerobic conditions
pH Tolerance:
- Metabolic activity maintained across a wide range of pH (4 to 9)
- Allows survival in various sections of the gastrointestinal tract
Clinical Relevance
C. butyricum has significant clinical relevance, both as a therapeutic agent and occasionally as a pathogen:
Therapeutic Applications:
Probiotic Use:
- Widely used as a probiotic in Japan, South Korea, China, and increasingly in Europe
- The C. butyricum MIYAIRI 588 strain (CBM588) is the most extensively studied probiotic strain
- Authorized for use as a food supplement in Europe
- Spore-forming nature provides superior shelf stability compared to non-spore-forming probiotics
Gastrointestinal Disorders:
- Treatment of antibiotic-associated diarrhea
- Management of inflammatory bowel diseases
- Alleviation of irritable bowel syndrome symptoms
- Protectin D1 production provides anti-inflammatory effects[1][5]
Immune Modulation:
- Stimulation of IL-10-producing macrophages, promoting anti-inflammatory responses
- Enhancement of gut-associated lymphoid tissue function
- Bifidogenic effects through expansion of IL-17A-producing γδ T cells
Cancer Immunotherapy Enhancement (CBM588):
A groundbreaking application of C. butyricum has emerged in cancer immunotherapy. The CBM588 strain has shown remarkable ability to enhance the efficacy of immune checkpoint inhibitors (ICIs):
Landmark Randomized Clinical Trial (Dizman et al., Nature Medicine 2022)[2][3]:
- Study Design: 30 patients with metastatic renal cell carcinoma randomized 2:1 to receive nivolumab-ipilimumab with or without CBM588
- Progression-Free Survival: 12.7 months vs 2.5 months (HR 0.15, 95% CI 0.05-0.47, P < 0.001) - a >5-fold improvement
- Response Rate: 58% with CBM588 vs 20% without (P = 0.06)
- Disease Control Rate: 79% with CBM588 vs 40% without
- Safety: No significant difference in toxicity between arms
- Mechanism: Responders showed increases in Bifidobacterium longum and Butyricimonas faecalis, and decreases in Desulfovibrio species
- Cytokine Changes: CBM588 increased chemokines including CXCL9, CXCL10, CCL2, and CCL4 that recruit cytotoxic T cells
Lung Cancer Studies[3][4]:
- Retrospective study in non-small cell lung cancer (NSCLC) showed profound impact on both PFS and overall survival
- Benefits were more pronounced in patients who had received antibiotic therapy
- Supports the mechanism that CBM588 can restore microbiome function compromised by antibiotics
Ongoing Clinical Trials:
- NCT05122546: Phase 2/3 study of nivolumab and cabozantinib with or without CBM588 in kidney cancer
- Multiple trials investigating CBM588 in various solid tumors treated with immunotherapy
Proposed Mechanisms for ICI Enhancement:
Bifidogenic Effects: Increases abundance of Bifidobacterium species associated with ICI response
Butyrate-Mediated Immune Modulation: Butyrate enhances anti-tumor immunity
Chemokine Upregulation: Increases T cell recruitment chemokines (CXCL9, CXCL10)
Regulatory T Cell Modulation: Prevents expansion of immunosuppressive Treg populations
Microbiome Restoration: Counters antibiotic-induced dysbiosis that impairs ICI response
Vaccine Development:
- Potential use as a vector for oral vaccine delivery against rotavirus and cholera
- Genetically engineered strains can deliver antigens to the gut, providing needle-free immunization
Aquaculture Applications:
- Improves growth performance and health benefits in aquatic animals
- Enhances disease resistance against pathogens like Vibrio parahaemolyticus
Pathogenic Potential:
Bacteremia:
- Rare cases of C. butyricum bacteremia, particularly in immunocompromised patients
- Prevalence estimated at 0.08% among bacteremia cases
- Most cases associated with underlying gastrointestinal pathology
Neurotoxin Production:
- Some environmental strains can produce botulinum neurotoxin type E
- These neurotoxin-producing strains have been associated with infant botulism
- Important: The MIYAIRI 588 (CBM588) probiotic strain lacks toxin genes and is considered safe[4][7]
Diagnostic Considerations:
- Identification through anaerobic culture, biochemical testing, and molecular methods
- Differentiation from other Clostridium species is important for proper management
- Toxin gene screening essential for strain characterization
Interactions with Other Microorganisms
C. butyricum engages in various interactions with other microorganisms in the gut ecosystem:
Promotion of Beneficial Bacteria:
- Increases the abundance of beneficial bacteria like Lactobacillus and Bifidobacterium
- Creates a more favorable gut environment for probiotic species
Inhibition of Pathogens:
- Antagonistic effects against pathogens such as Vibrio parahaemolyticus
- Competitive exclusion of harmful bacteria through occupation of ecological niches
- Production of antimicrobial substances that inhibit pathogen growth
Synergistic Relationships:
- Works synergistically with prebiotics like galactooligosaccharides (GOS) and resistant starch (RS)
- Combined with prebiotics, it shows enhanced effects on SCFA production and beneficial modulation of gut microbiota
Microbiome Restoration:
- Helps restore balance to dysbiotic microbiomes, such as after antibiotic treatment
- Facilitates the return to a healthier microbial community structure
Cross-Feeding:
- Metabolic products of C. butyricum can serve as substrates for other beneficial bacteria
- Participates in complex metabolic networks within the gut ecosystem
Research Significance
C. butyricum has significant research importance across multiple scientific disciplines:
Immuno-Oncology Research (Most Significant Emerging Area):
- Microbiome-Immunotherapy Axis: C. butyricum CBM588 has emerged as a paradigm-shifting intervention in cancer immunotherapy[5][3]
- Understanding how gut microbiome composition influences response to immune checkpoint inhibitors
- Investigation of the mechanisms by which butyrate-producing bacteria enhance anti-tumor immunity
- The gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors[6][8]
- Bifidobacterium species promote antitumor immunity and facilitate anti-PD-L1 efficacy[7][10]
Probiotic Research:
- Model organism for studying probiotic mechanisms and effects
- Investigation of strain-specific properties and their health implications
- Development of next-generation probiotics with enhanced benefits
- Understanding spore germination and colonization mechanisms
Gut-Brain Axis Studies:
- Research into the connection between butyrate production and mental health
- Investigation of potential protective effects against depression and other neurological conditions
- Exploration of metabolite-mediated communication between gut and brain
Immunology:
- Study of immune modulation through gut microbiota
- Understanding of how butyrate-producing bacteria influence systemic immunity
- Research on T cell differentiation and function modulation
- Investigation of chemokine networks influenced by gut bacteria
Biotechnology:
- Development of C. butyricum as a vector for vaccine delivery
- Genetic engineering applications for therapeutic purposes
- Production of butyrate and other valuable metabolites
- Development of live biotherapeutic products (LBPs)
Aquaculture and Agriculture:
- Research on improving animal health and growth through probiotic supplementation
- Development of alternatives to antibiotics in animal husbandry
- Studies on pathogen inhibition (e.g., Vibrio parahaemolyticus, E. coli O157:H7)[8][12]
Clinical Microbiology:
- Investigation of the dual nature of C. butyricum as both beneficial and potentially pathogenic
- Development of diagnostic methods for differentiating between beneficial and harmful strains
- Strain characterization and toxin gene screening methodologies
References
Liang, H., Tran, N. T., Deng, T., et al. (2023). Identification and Characterization of a Potential Probiotic, Clostridium butyricum G13, Isolated from the Intestine of the Mud Crab (Scylla paramamosain). Microbiology Spectrum, 11(4).
Omorotionmwan, B. (2024). Gut microbiome: meet Clostridium butyricum – the bacteria that helps keep us feeling our best. The Conversation.
Cassir, N., Benamar, S., & La Scola, B. (2016). Clostridium butyricum: from beneficial to a new emerging pathogen. Clinical Microbiology and Infection, 22(1), 37-45.
Luo, Y., Zhang, L., Li, H., et al. (2019). Different Types of Dietary Fibers Trigger Specific Alterations in Composition and Predicted Functions of Colonic Bacterial Communities in BALB/c Mice. Frontiers in Microbiology, 10, 1689.
Hagihara, M., Yamashita, R., Matsumoto, A., et al. (2018). The impact of probiotic Clostridium butyricum MIYAIRI 588 on murine gut metabolic alterations. Journal of Infection and Chemotherapy, 24(8), 627-634.
Cassir, N., Benamar, S., & La Scola, B. (2016). Clostridium butyricum: from beneficial to a new emerging pathogen. Clinical Microbiology and Infection, 22(1), 37-45.
Hagihara, M., Yamashita, R., Matsumoto, A., et al. (2018). The impact of probiotic Clostridium butyricum MIYAIRI 588 on murine gut metabolic alterations. Journal of Infection and Chemotherapy, 24(8), 627-634.
Luo, Y., Zhang, L., Li, H., et al. (2019). Different Types of Dietary Fibers Trigger Specific Alterations in Composition and Predicted Functions of Colonic Bacterial Communities in BALB/c Mice. Frontiers in Microbiology, 10, 1689.