Neisseria subflava
Overview
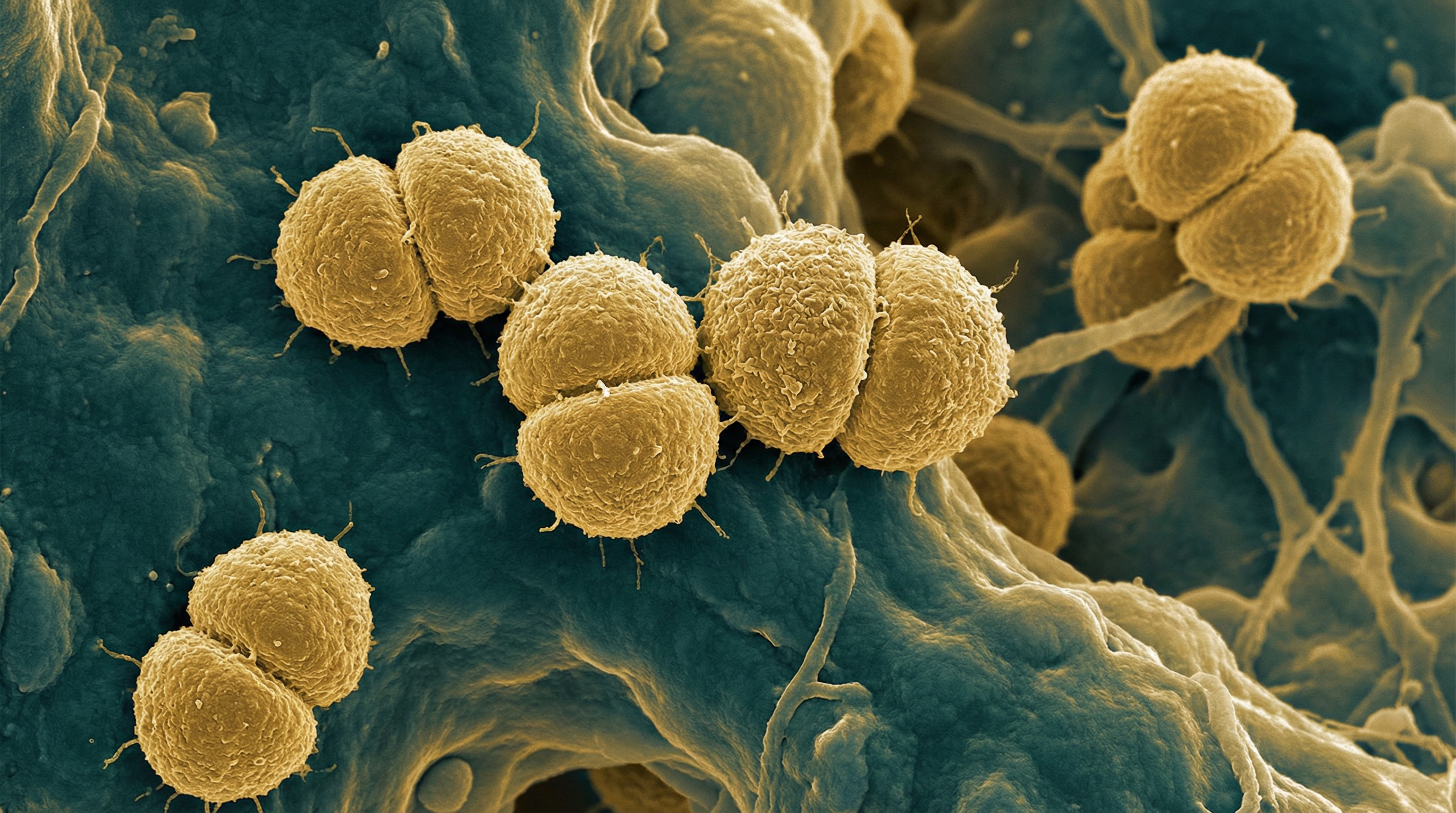
Neisseria subflava is a Gram-negative, aerobic diplococcus that belongs to the family Neisseriaceae within the phylum β-Proteobacteria. It is a commensal organism that primarily colonizes the human oral cavity, nasopharynx, and upper respiratory tract. N. subflava represents a consolidated taxonomic entity, as recent genomic approaches have led to the reclassification of previously separate biovars (N. subflava biovar subflava, perflava, flava, and N. flavescens) into a single species. As a transient, low-abundance member of the human microbiome, N. subflava typically exists as part of the normal flora without causing disease in healthy individuals. However, it can act as an opportunistic pathogen in certain circumstances, particularly in immunocompromised hosts or when introduced to normally sterile sites. Recent research has identified potential roles for N. subflava in respiratory conditions such as bronchiectasis, where it may contribute to epithelial damage and inflammation. Despite being less studied than pathogenic Neisseria species like N. meningitidis and N. gonorrhoeae, N. subflava plays an important ecological role in the human microbiome and may influence the colonization and behavior of other microorganisms in the respiratory tract.
Characteristics
Neisseria subflava exhibits several distinctive characteristics that define its biological identity:
- Morphology: Gram-negative diplococci (pairs of cocci) with adjacent sides flattened, giving a coffee bean appearance when viewed microscopically
- Cell wall structure: Typical Gram-negative cell envelope with an outer membrane containing lipooligosaccharide (LOS)
- Oxygen requirements: Strictly aerobic, requiring oxygen for growth
- Growth conditions: Optimal growth at 35-37°C in a humid environment with 5-10% CO2
- Motility: Non-motile
- Spore formation: Non-spore forming
- Colonial appearance: Forms small, round, smooth, glistening, convex colonies that are typically grayish-white to yellowish in color
- Biochemical properties:
- Oxidase-positive
- Catalase-positive
- Limited carbohydrate metabolism compared to other bacteria
- Capable of fermenting glucose, maltose, and sometimes sucrose
- Unable to reduce nitrate to nitrite (distinguishing it from some other Neisseria species)
- Genetic characteristics:
- Moderate-sized genome (approximately 2.0-2.5 Mb)
- Distinct rplF gene alleles (alleles 16, 62, 69, 70, 79, and 146 have been identified)
- Genomic evidence of horizontal gene transfer with other Neisseria species
- Taxonomic consolidation: Modern genomic approaches have led to the consolidation of previously separate biovars (N. subflava biovar subflava, perflava, flava, and N. flavescens) into a single species
N. subflava can be distinguished from other Neisseria species through a combination of biochemical tests, including patterns of acid production from carbohydrates, and molecular methods such as 16S rRNA sequencing or analysis of the rplF gene. However, definitive identification often requires whole genome sequencing due to the close genetic relationships among commensal Neisseria species. The species shows considerable genetic diversity, which may reflect its adaptation to different microenvironments within the human upper respiratory tract.
Role in Human Microbiome
Neisseria subflava occupies a specific ecological niche within the human microbiome:
- Primary habitat: Predominantly found in the oral cavity, nasopharynx, and upper respiratory tract
- Prevalence: Present in a high percentage of healthy individuals, with studies suggesting it may be one of the most prevalent commensal Neisseria species
- Abundance: Typically exists as a low-abundance member of the microbiome, constituting less than 2% of the total bacterial population in colonized sites
- Developmental trajectory: Present throughout life, though abundance may vary with age and health status
- Ecological role: Functions as a commensal organism that contributes to the complex microbial community of the upper respiratory tract
- Co-colonization patterns: Often co-exists with other commensal bacteria including streptococci, Haemophilus species, and other Neisseria species
- Biofilm participation: Can participate in multispecies biofilms in the oral cavity and respiratory tract
- Temporal stability: Considered a transient colonizer, with population levels that may fluctuate over time
- Spatial distribution: May show preferences for specific microenvironments within the upper respiratory tract
The ecological significance of N. subflava in the human microbiome is not fully understood, but it likely contributes to the overall microbial balance in the upper respiratory tract. Some studies suggest that commensal Neisseria species, including N. subflava, may play a role in modulating the colonization and behavior of pathogenic Neisseria species, particularly N. meningitidis. This modulation could occur through competition for nutrients and attachment sites, production of inhibitory compounds, or influence on host immune responses. Additionally, as part of the normal microbiota, N. subflava may occupy ecological niches that might otherwise be colonized by more pathogenic organisms, potentially contributing to colonization resistance against respiratory pathogens.
Health Implications
Neisseria subflava has several implications for human health, functioning primarily as a commensal but occasionally as an opportunistic pathogen:
Commensal role:
- As part of the normal microbiota, may contribute to colonization resistance against more pathogenic organisms
- May play a role in educating and modulating the immune system in the respiratory tract
- Potential competitive inhibition of pathogenic Neisseria species, including N. meningitidis
Opportunistic infections:
- Rarely causes disease in immunocompetent individuals
- Can cause opportunistic infections in immunocompromised hosts
- Has been isolated from cases of endocarditis, particularly in individuals with underlying heart conditions
- Occasionally implicated in cases of meningitis, though much less frequently than N. meningitidis
- Reported in some cases of bacteremia, particularly in individuals with indwelling medical devices
Respiratory conditions:
- Recent research has identified potential roles in bronchiectasis, where N. subflava has been associated with poor clinical outcomes
- Studies suggest it may promote loss of epithelial completeness and integrity in the respiratory tract
- May contribute to inflammatory processes in the lungs under certain conditions
- Has been isolated from patients with chronic obstructive pulmonary disease (COPD) exacerbations
Interactions with other conditions:
- Potential modulator of other respiratory infections
- May influence the severity or course of conditions like asthma or cystic fibrosis, though evidence is limited
- Possible contributor to oral health conditions as part of complex biofilms
Antibiotic resistance:
- Generally susceptible to antibiotics commonly used for respiratory infections
- However, increasing reports of beta-lactamase production in some strains
- Potential reservoir of resistance genes that could be transferred to more pathogenic species
The health significance of N. subflava is context-dependent, with its impact varying based on host factors, co-infecting organisms, and environmental conditions. In most healthy individuals, it exists as a harmless commensal, but in specific circumstances—particularly in immunocompromised hosts or when introduced to normally sterile sites—it can cause disease. Recent research suggesting its potential role in bronchiectasis and other respiratory conditions highlights the need for further investigation into the mechanisms by which this typically commensal organism may contribute to pathological processes.
Metabolic Activities
Neisseria subflava exhibits specific metabolic capabilities adapted to its ecological niche in the human upper respiratory tract:
Carbohydrate metabolism:
- Oxidatively metabolizes glucose and maltose
- Some strains can utilize sucrose
- Generally limited carbohydrate utilization compared to many other bacteria
- Produces acid from carbohydrate metabolism but not gas
- Lacks the ability to ferment lactose, distinguishing it from some other Neisseria species
Respiratory metabolism:
- Strictly aerobic, utilizing oxygen as the terminal electron acceptor
- Contains cytochrome oxidase, giving a positive oxidase test
- Possesses catalase, which decomposes hydrogen peroxide
- Unlike some Neisseria species, cannot use nitrate as an alternative electron acceptor
Nitrogen metabolism:
- Unable to reduce nitrate to nitrite
- Can utilize certain amino acids as nitrogen sources
- Limited capacity for amino acid biosynthesis, likely requiring environmental sources
Lipid metabolism:
- Produces lipases that may contribute to nutrient acquisition
- Synthesizes lipooligosaccharide (LOS) as a major component of its outer membrane
- LOS structure differs from the lipopolysaccharide (LPS) of many other Gram-negative bacteria
Enzyme production:
- Produces oxidase and catalase
- Some strains produce IgA1 protease, which may contribute to colonization
- Various peptidases for protein utilization
- Limited production of extracellular enzymes compared to many pathogens
Adaptation to the respiratory environment:
- Metabolic capabilities suited to the nutrient availability in the upper respiratory tract
- Able to utilize host-derived compounds, including potentially mucin components
- Metabolic flexibility allowing survival in the dynamic environment of the respiratory mucosa
Biofilm metabolism:
- Altered metabolic activity when growing in biofilms
- Potential metabolic interactions with other microorganisms in multispecies biofilms
- Production of extracellular matrix components during biofilm formation
Stress responses:
- Metabolic adaptations to survive oxidative stress
- Mechanisms to cope with nutrient limitation
- Potential metabolic dormancy under unfavorable conditions
The metabolic capabilities of N. subflava are relatively modest compared to many other bacteria, reflecting its adaptation to a specific niche as a commensal organism. Its limited carbohydrate utilization profile and inability to reduce nitrate are useful characteristics for distinguishing it from other Neisseria species in laboratory identification. The metabolic activities of N. subflava likely contribute to its ability to persist in the human upper respiratory tract and may influence its interactions with other members of the respiratory microbiome.
Clinical Relevance
Neisseria subflava has several aspects of clinical significance:
Diagnostic considerations:
- May be isolated from respiratory specimens, where it is typically considered part of the normal flora
- Can be misidentified as other Neisseria species, including pathogenic N. meningitidis, if identification methods are limited
- Definitive identification often requires molecular methods or whole genome sequencing
- Presence in normally sterile sites (blood, cerebrospinal fluid) should be considered potentially significant
- Laboratory identification challenges due to similarities with other Neisseria species
Opportunistic infections:
- Rare cause of endocarditis, particularly in individuals with underlying heart conditions
- Occasional reports of meningitis, though much less common than with N. meningitidis
- Cases of bacteremia, often associated with indwelling medical devices
- Respiratory infections, particularly in immunocompromised hosts
- Potential contributor to polymicrobial infections
Respiratory disease associations:
- Emerging evidence for a role in bronchiectasis, with recent studies showing associations with poor clinical outcomes
- Potential contributor to exacerbations of chronic respiratory conditions
- May promote epithelial damage in the respiratory tract
- Possible involvement in inflammatory processes in the lungs
Antimicrobial susceptibility:
- Generally susceptible to antibiotics commonly used for respiratory infections
- Some strains produce beta-lactamases, conferring resistance to certain penicillins
- Potential reservoir of antimicrobial resistance genes
- Susceptibility testing not routinely performed due to infrequent pathogenic role
- Treatment of infections typically based on empirical therapy
Public health significance:
- Not considered a significant public health threat
- No vaccine available or under development
- Not subject to surveillance or reporting requirements
- May serve as a reservoir for genetic exchange with pathogenic Neisseria species
Emerging research areas:
- Potential role in modulating colonization by pathogenic Neisseria species
- Possible applications in developing strategies to prevent meningococcal disease
- Investigation of mechanisms by which it may contribute to respiratory pathology
- Exploration of its role in the respiratory microbiome and implications for health
The clinical relevance of N. subflava is primarily as an opportunistic pathogen that rarely causes disease in immunocompetent individuals. However, its potential role in respiratory conditions like bronchiectasis is an emerging area of research that may change our understanding of its clinical significance. From a diagnostic perspective, accurate identification is important to distinguish it from pathogenic Neisseria species, particularly in specimens from normally sterile sites. While not a major focus of clinical microbiology, awareness of N. subflava's potential to cause opportunistic infections and its possible role in respiratory pathology is relevant for comprehensive patient care.
Interactions with Other Microorganisms
Neisseria subflava engages in various interactions with other members of the human microbiome:
Interactions with other Neisseria species:
- May compete with pathogenic Neisseria species, particularly N. meningitidis, for resources and attachment sites
- Potential horizontal gene transfer with other Neisseria species, contributing to genetic diversity
- Possible modulation of virulence gene expression in pathogenic Neisseria through quorum sensing or other mechanisms
- May influence the establishment and persistence of N. meningitidis colonization
Biofilm participation:
- Forms part of multispecies biofilms in the oral cavity and respiratory tract
- Contributes to biofilm structure and function through production of extracellular matrix components
- Engages in metabolic cooperation with other biofilm members
- May influence biofilm resistance to antimicrobials and host defense mechanisms
Interactions with streptococci:
- Co-exists with various Streptococcus species in the upper respiratory tract
- Potential metabolic cross-feeding relationships
- May influence streptococcal biofilm formation and stability
- Possible competitive or cooperative interactions depending on environmental conditions
Interactions with other respiratory commensals:
- Cohabits with Haemophilus species, Moraxella catarrhalis, an (Content truncated due to size limit. Use line ranges to read in chunks)
