Roseburia intestinalis
Overview
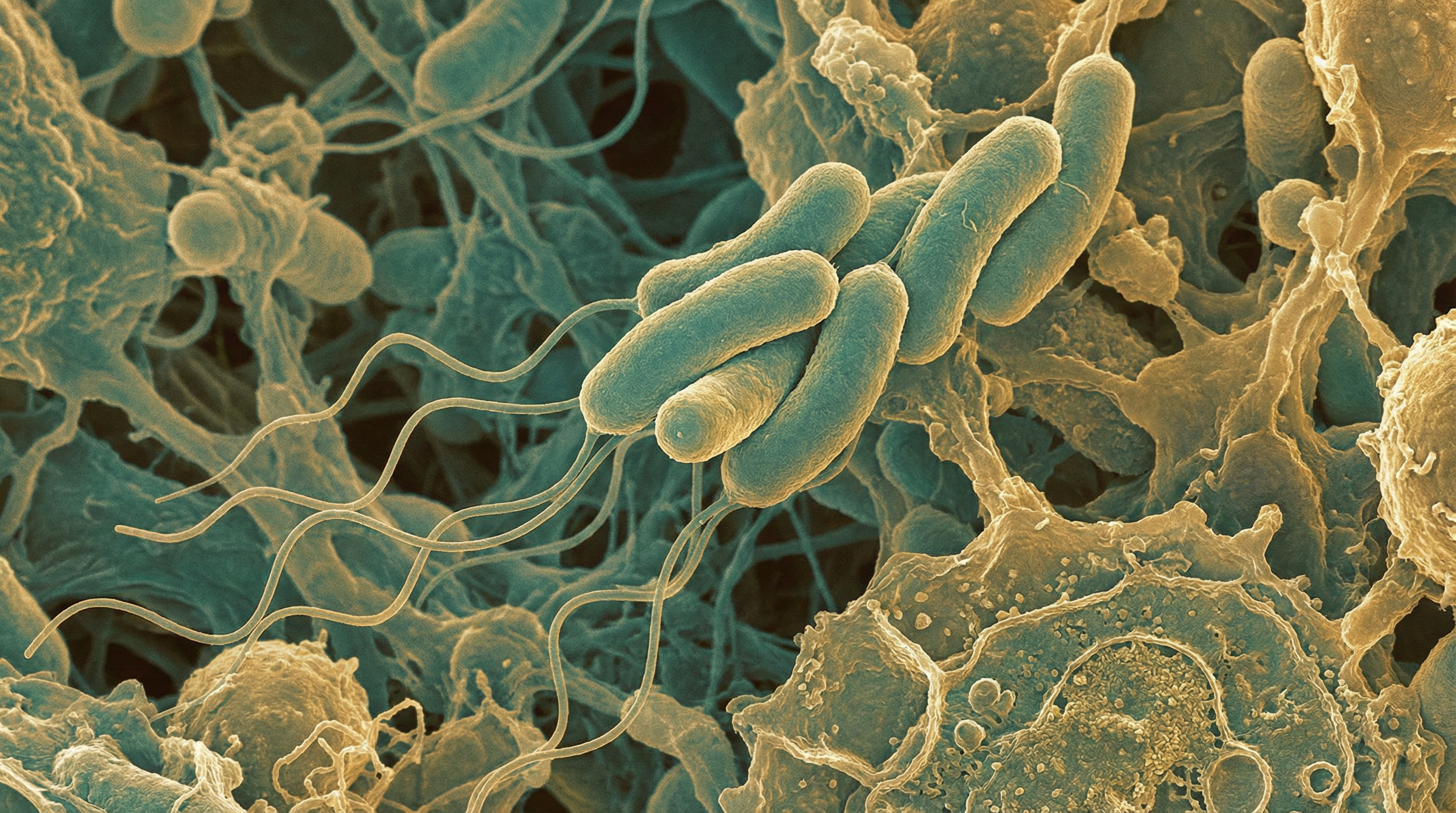
Roseburia intestinalis is an anaerobic, Gram-positive, slightly curved rod-shaped flagellated bacterium that belongs to the phylum Firmicutes, class Clostridia, order Clostridiales, and family Lachnospiraceae. It is one of the most abundant butyrate-producing bacteria in the human gut microbiome, typically accounting for 0.9%–5.0% (mean = 2.3%) of the total microbiota, with Roseburia species collectively representing 7-24% of total bacteria in the healthy human colon. R. intestinalis is increasingly recognized as a key beneficial microorganism with significant potential as a "next-generation probiotic" due to its numerous positive effects on human health.
Characteristics
R. intestinalis is an obligate anaerobe that requires specific growth conditions. It produces butyric acid as a major metabolic end product through the butyryl-CoA:acetate CoA-transferase pathway, with enzyme activity measured at 38.95 ± 3.40 µmol⁻¹ mg protein⁻¹ min⁻¹ during exponential growth phase. The bacterium possesses flagella that allow it to penetrate the colonic mucus layer and interact with the epithelium.
Key features include:
- Morphology: Slightly curved rod-shaped, flagellated bacteria
- Metabolism: Obligate anaerobe that produces butyrate (~2000 µg/mL in culture) as a major end product of fermentation
- Growth Requirements: Anaerobic conditions at 37°C with specific growth rate of 0.44 ± 0.05 h⁻¹
- Genome Features: Contains butyryl-CoA:acetate CoA transferase, the key enzyme for butyrate production; 4,340 coding sequences; 41 genomic islands; 7 prophages
- Substrate Utilization: Can ferment dietary fibers, especially resistant starches, xylan, and β-mannan
β-Mannan Metabolism: A Specialized Capability
R. intestinalis demonstrates remarkable specialization in degrading dietary β-mannans, plant cell wall polysaccharides commonly found in human diets. The bacterium expresses two conserved loci (MULL and MULS) containing specialized enzymatic machinery:
- RiGH26: An extracellular endomannanase that hydrolyzes spruce acetyl-galactoglucomannan (AcGGM) into oligosaccharides within 1 hour
- ABC Transporter System: RiMnBP/RiMPP1/RiMPP2 for internalization (binding affinity Kd = 2.55 µM)
- Decorations Removal: RiCE2 and RiCEX esterases remove acetyl groups; RiGH36 α-galactosidase removes galactose with 100% efficiency
In competitive co-culture experiments, R. intestinalis outcompeted Bacteroides ovatus (72.5% vs 27.5% at stationary phase), demonstrating its role as a primary, specialist degrader. Dietary supplementation with 2.5-7.5% AcGGM produces a 10-30 fold increase in R. intestinalis relative abundance.
Gut Barrier and Epithelial Health
R. intestinalis exerts profound protective effects on gut barrier function through multiple mechanisms:
Tight Junction Enhancement
Studies demonstrate upregulation of critical tight junction proteins including ZO-1, claudin-1, claudin-3, occludin, and TJP1. In 27-OHC-induced cognitive impairment models, R. intestinalis reversed reductions in occludin (F = 24.33, p < 0.0001) and claudin-1 (F = 21.4, p < 0.0001).
Intestinal Permeability
Using FITC-dextran (4 kDa) permeability assays, R. intestinalis significantly reduced intestinal permeability in ApcMin/+ mice and ethanol-challenged Caco-2 cells (p < 0.001).
Mucus Layer Protection
R. intestinalis colonizes the cecum and colonic mucus layers, upregulating secreted mucin MUC2 and preventing endotoxin (LPS) translocation.
Anti-Inflammatory and Immunomodulatory Effects
Flagellin-TLR5 Signaling Pathway
R. intestinalis flagellin binds to Toll-like receptor 5 (TLR5) on intestinal epithelial cells, initiating a protective immunomodulatory cascade:
- TSLP Production: Flagellin-TLR5 interaction induces thymic stromal lymphopoietin (TSLP) secretion by IECs
- Dendritic Cell Activation: TSLP activates dendritic cells to secrete IL-10 and TGF-β (>1000-fold increase vs IEC supernatant alone)
- Treg Differentiation: This promotes CD4+CD25+FOXP3+ regulatory T cell differentiation
Importantly, IEC-expressed TLR5—not immune cell TLR5—is critical for these protective effects, as demonstrated by bone marrow transplantation experiments. In Crohn's disease patients (24 CD vs 22 healthy controls), R. intestinalis levels positively correlated with TLR5, TSLP, TGF-β, and IL-10 expression (p<0.01 or p<0.001).
NLRP3 Inflammasome Inhibition
R. intestinalis flagellin upregulates miR-223-3p, which targets the NLRP3 3'-UTR, resulting in:
- Reduced NLRP3 inflammasome activation
- Decreased caspase-1 cleavage
- Inhibition of Gasdermin D-mediated pyroptosis
- Reduced IL-1β and IL-18 secretion
In DSS-induced colitis models, flagellin treatment (50 mg/kg IP daily) significantly restored body weight, decreased disease activity index scores, and inhibited serum IL-1β, IL-18, TNF-α, and IL-6 (p<0.05).
Cancer Prevention and Immunotherapy Enhancement
Colorectal Cancer Protection
R. intestinalis is significantly depleted in CRC patients (444 CRC vs 575 healthy controls across 5 cohorts). The bacterium provides protection through multiple mechanisms:
Butyrate/TLR5/NF-κB Pathway in CD8+ T Cells:
- Butyrate binds TLR5 on CD8+ T cells (Surface Plasmon Resonance affinity: 264 μM)
- Activates NF-κB signaling with nuclear translocation of p65
- Enhances production of granzyme B, IFN-γ, and TNF-α
- TLR5 expression increases >10-fold in R. intestinalis-treated CD8+ T cells
Radiosensitization via OR51E1/RALB Autophagy: R. intestinalis butyrate activates the G-protein-coupled receptor OR51E1, triggering RALB signaling and autophagy-mediated CRC cell death. G2/M arrest increased from 24.9% (control) to 30.88% (radiation) to 37.52% (radiation + butyrate).
Anti-PD-1 Immunotherapy Enhancement
Perhaps most remarkably, R. intestinalis administration significantly improves anti-PD-1 efficacy in microsatellite instability-low (MSI-low) and microsatellite stable (MSS) colorectal tumors—tumors typically resistant to checkpoint immunotherapy. This effect is mediated through enhanced cytotoxic CD8+ T cell infiltration and activation.
Metabolic Health Benefits
Type 2 Diabetes
R. intestinalis serves as a marker for classifying type 2 diabetes, being consistently depleted in T2DM patients across 345 individuals from multiple global cohorts. The bacterium increases IL-22 production, which restores insulin sensitivity and alleviates diabetes.
Liver Disease
In a twin cohort study (410 Korean twins), Roseburia abundance was inversely associated with alcohol consumption (r=-0.0005, p=0.0002). Administration improved survival in chronic alcoholic liver disease from 37.5% to 66.7%. Roseburia abundance also inversely correlates with liver fibrosis and AST levels.
For NAFLD, Ruminococcaceae (including Roseburia) shows inverse correlation with fibrosis in non-obese patients (p=0.0012), with microbiome-metabolite combination models achieving AUC=0.939 for predicting significant fibrosis.
Cognitive Health and Gut-Brain Axis
R. intestinalis demonstrates neuroprotective effects through the gut-brain axis. In 27-OHC-induced cognitive impairment models:
- Novel Object Recognition Index improved (p=0.0132)
- Y Maze Spontaneous Alternation improved (p=0.0011)
- Morris Water Maze platform crossings increased (p=0.0089)
- Restored PSD-95 and BDNF expression
- Normalized brain m6A methylation (F=12.14, p=0.0028)
Safety Profile and Probiotic Potential
Comprehensive safety assessment of R. intestinalis L1-82 demonstrates an excellent safety profile:
| Parameter | Result |
|---|---|
| LD50 | >1.9 × 10⁹ CFU/kg (exceeds highest dose tested) |
| NOAEL | 1.32 × 10⁹ CFU/kg/day for 28 days |
| Cytotoxicity | None observed in NCM460, HT-29, Caco-2 cells |
| Hemolytic activity | Negative |
| Gelatinase activity | Negative |
| Virulence factors | None detected (VFDB screening) |
Antibiotic Susceptibility
R. intestinalis is sensitive to ampicillin, vancomycin, gentamicin, erythromycin, clindamycin, tetracycline, and chloramphenicol, meeting EFSA safety requirements.
Formulation Challenges
As a strictly anaerobic organism, R. intestinalis presents manufacturing challenges:
- Requires oxygen-free cultivation and storage
- Limited shelf-life compared to aerotolerant probiotics
- Must survive gastric transit to reach the colon
Potential solutions include microencapsulation, synbiotic formulations with β-mannan or resistant starch prebiotics, and prebiotic strategies to promote endogenous populations.
Disease Associations Summary
R. intestinalis depletion is consistently observed in:
- Inflammatory bowel disease (Crohn's disease and ulcerative colitis)
- Colorectal cancer
- Type 2 diabetes and metabolic syndrome
- Alcoholic and non-alcoholic liver disease
- Cardiovascular disease and atherosclerosis
- Severe COVID-19 (correlates with elevated inflammatory markers)
- Cognitive impairment
Future Therapeutic Applications
R. intestinalis represents a promising next-generation probiotic with potential applications in:
- Combination with immunotherapy: Enhancing anti-PD-1 efficacy in previously resistant CRC
- Radiosensitization: Improving radiotherapy outcomes while protecting healthy tissue
- Synbiotic formulations: Paired with β-mannan or resistant starch prebiotics
- Precision medicine: Biomarker-guided administration to patients with confirmed depletion
- Cognitive health: Targeting gut-brain axis dysfunction in neurological conditions
