Streptococcus pneumoniae
Key Characteristics
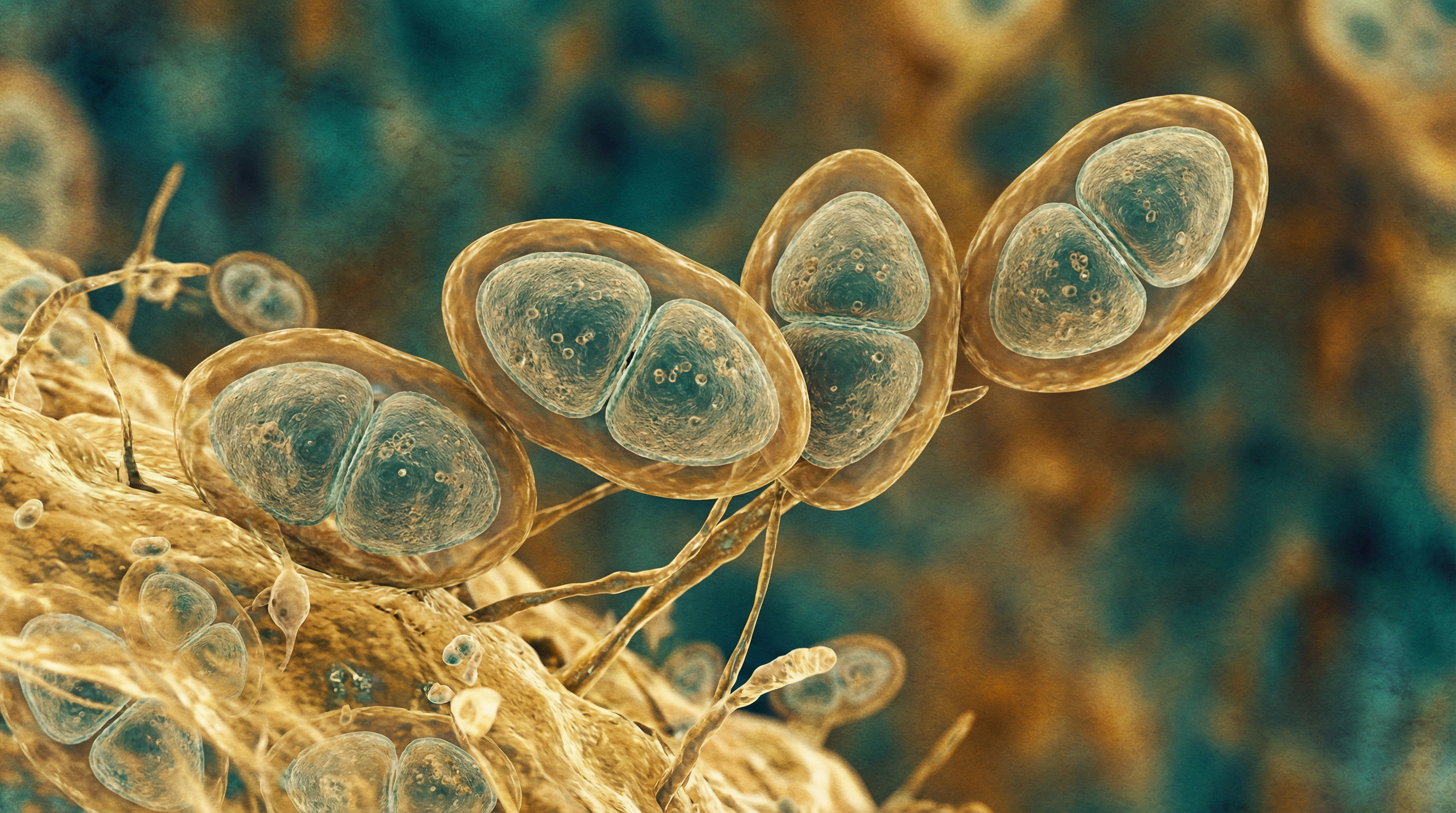
Streptococcus pneumoniae, commonly known as pneumococcus, is a significant human pathogen with several distinctive characteristics:
- Gram-positive, lancet-shaped (diplococcal) bacteria that typically occur in pairs or short chains
- Facultative anaerobic organism that can grow in environments with or without oxygen
- Alpha-hemolytic on blood agar, producing a characteristic green zone around colonies
- Encapsulated with a polysaccharide capsule that is a major virulence factor
- More than 100 distinct serotypes based on capsular polysaccharide structure
- Naturally competent for genetic transformation, readily taking up DNA from the environment
- Possesses numerous surface proteins that mediate host-pathogen interactions
- Contains cell wall with teichoic acid and lipoteichoic acid that trigger inflammatory responses
- Produces pneumolysin, a pore-forming cytotoxin that damages host cells
- Expresses IgA1 protease that cleaves human immunoglobulin A
- Capable of forming biofilms during colonization
- Exhibits phase variation between transparent and opaque colony phenotypes
- Transparent variants predominate during colonization, while opaque variants are associated with invasive disease
- Possesses multiple adhesins for attachment to host epithelial cells
- Contains choline-binding proteins that anchor to the cell wall
- Expresses neuraminidase enzymes that cleave sialic acid from host glycoproteins
- Produces hydrogen peroxide as a metabolic byproduct, which can damage host tissues
- Capable of autolysis through the action of autolysin enzymes
- Susceptible to optochin, which is used as a diagnostic test
- Soluble in bile salts, another distinguishing diagnostic feature
- Ferments various carbohydrates, producing primarily lactic acid
- Lacks catalase enzyme, making it sensitive to oxidative stress
- Possesses multiple transport systems for nutrient acquisition
- Contains two-component regulatory systems that control virulence gene expression
- Exhibits complex quorum-sensing mechanisms that regulate population behavior
- Demonstrates significant genomic plasticity through horizontal gene transfer
- Capable of developing antibiotic resistance through genetic recombination
- Requires complex media containing blood or serum for laboratory cultivation
- Optimal growth temperature of 35-37°C, corresponding to human body temperature
- Sensitive to desiccation in the environment, requiring host-to-host transmission
- Possesses multiple proteases that contribute to virulence and tissue invasion
- Contains pili structures in some strains that enhance adherence and colonization
- Exhibits strain-specific variations in virulence and tissue tropism
Streptococcus pneumoniae represents a highly adapted human pathogen that has evolved sophisticated mechanisms for colonization, immune evasion, and disease causation. Its remarkable genetic adaptability and diverse virulence factors contribute to its success as both a commensal organism and a significant cause of morbidity and mortality worldwide.
Role in Human Microbiome
Streptococcus pneumoniae occupies a complex niche within the human microbiome, primarily as a colonizer of the upper respiratory tract:
Nasopharyngeal Colonization:
- Primary ecological niche is the human nasopharynx
- Colonization rates vary significantly by age and setting:
- 5-10% of adults without children
- 20-60% of school-aged children
- 50-60% of individuals in crowded settings (military installations, daycare centers)
- Asymptomatic carriage is common and represents the reservoir for transmission
- Duration of carriage varies, generally longer in children than adults
- Colonization is a prerequisite for both transmission and invasive disease
- Seasonal variations with higher carriage rates during winter months
- Multiple serotypes can colonize simultaneously, though uncommon
- Carriage induces serotype-specific immunity that influences future colonization
Interactions with Respiratory Microbiome:
- Complex relationships with other members of the nasopharyngeal microbiota
- Competitive interactions with other streptococcal species, particularly Streptococcus viridans group
- Antagonistic relationship with Staphylococcus aureus in many individuals
- Synergistic relationships with certain viral pathogens, especially influenza virus
- Competition for nutrients and attachment sites with other respiratory commensals
- Production of hydrogen peroxide that inhibits growth of some competing bacteria
- Bacteriocin production that can suppress growth of closely related species
- Biofilm formation that involves multi-species communities
- Altered microbiome composition during pneumococcal colonization
- Reduced microbial diversity associated with pneumococcal dominance
Microenvironmental Adaptations:
- Preferentially resides in the mucus layer overlying the epithelial surface
- Adapts to the relatively nutrient-poor environment of the nasopharynx
- Utilizes host glycans as carbon sources when free sugars are limited
- Expresses different genes during colonization versus invasion
- Transparent colony phenotype predominates during colonization
- Downregulates capsule expression during initial colonization to enhance adherence
- Upregulates capsule production when faced with host immune responses
- Forms biofilms that provide protection from environmental stresses
- Exhibits metabolic flexibility to adapt to changing nutrient availability
- Modifies surface structures to evade host immune recognition
Transition from Commensal to Pathogen:
- Capable of shifting from commensal to pathogenic relationship with host
- Local spread can lead to otitis media and sinusitis
- Aspiration can result in pneumonia
- Invasion of bloodstream leads to bacteremia and potentially meningitis
- Transition often triggered by viral co-infections that disrupt epithelial barriers
- Host factors such as age, immune status, and genetic background influence progression to disease
- Bacterial factors including serotype and virulence gene expression affect invasive potential
- Environmental factors such as smoking and air pollution increase risk of invasive disease
- Shift from colonization to invasion represents a "dead end" for transmission
Population Dynamics and Transmission:
- Transmitted through respiratory droplets requiring close contact
- Higher transmission rates in crowded settings and during respiratory virus seasons
- Viral co-infections increase bacterial shedding and transmission
- Pneumococcal vaccination has altered population dynamics through herd immunity
- Serotype replacement occurs following widespread vaccination
- Geographic variations in serotype distribution and prevalence
- Genetic recombination contributes to emergence of new variants
- Antibiotic use selects for resistant strains within the microbiome
- Carriage of multiple strains facilitates horizontal gene transfer
- Transmission chains often begin with children who spread to adults
Ecological Impact of Interventions:
- Pneumococcal conjugate vaccines have reduced vaccine-type colonization
- Ecological niche vacated by vaccine serotypes filled by non-vaccine serotypes
- Antibiotic use disrupts normal microbiota and can promote pneumococcal overgrowth
- Changes in pneumococcal population structure following vaccination
- Altered interactions with other microbiome members after interventions
- Potential for increased colonization by other pathogens following pneumococcal elimination
- Long-term ecological consequences of pneumococcal vaccination still being evaluated
- Microbiome restoration strategies being explored to prevent pathogen colonization
Streptococcus pneumoniae maintains a delicate balance between commensal colonization and pathogenic invasion within the human microbiome. Its interactions with other microorganisms and the host immune system shape its ecological niche and influence its potential to cause disease. Understanding these complex relationships is crucial for developing effective strategies to prevent pneumococcal disease while maintaining a healthy respiratory microbiome.
Health Implications
Streptococcus pneumoniae is associated with a spectrum of diseases ranging from mild to life-threatening:
Respiratory Tract Infections:
Pneumonia:
- Leading cause of community-acquired pneumonia worldwide
- Characterized by lung inflammation, consolidation, and alveolar infiltration
- Symptoms include fever, cough, chest pain, and difficulty breathing
- Can progress to respiratory failure in severe cases
- Higher mortality in elderly, immunocompromised, and those with comorbidities
- Often preceded by viral respiratory infections
- Radiographic findings typically show lobar consolidation
- May be complicated by parapneumonic effusion or empyema
Bronchitis:
- Inflammation of the bronchial tubes
- Less common presentation than pneumonia
- Often occurs as a secondary infection following viral illness
- Symptoms include productive cough and chest discomfort
- Generally less severe than pneumonia
Sinusitis:
- Inflammation of the paranasal sinuses
- Symptoms include facial pain, pressure, nasal congestion, and discharge
- May become chronic if inadequately treated
- Often polymicrobial with other respiratory bacteria
Otitis Media:
- Infection of the middle ear
- Particularly common in children
- Symptoms include ear pain, fever, and hearing impairment
- May lead to tympanic membrane perforation
- Recurrent infections can cause long-term hearing problems
- Often follows upper respiratory viral infections
Invasive Pneumococcal Disease (IPD):
Bacteremia/Septicemia:
- Presence of bacteria in the bloodstream
- Can occur with or without identifiable focus of infection
- Symptoms include high fever, chills, and systemic toxicity
- May lead to septic shock with hypotension and organ dysfunction
- Higher mortality rates than non-invasive disease
- Risk increases with extremes of age and immunocompromise
Meningitis:
- Inflammation of the meninges surrounding the brain and spinal cord
- Symptoms include headache, neck stiffness, altered mental status, and photophobia
- Medical emergency requiring prompt antibiotic treatment
- Significant mortality (20-30%) despite appropriate therapy
- Neurological sequelae common in survivors
- Highest incidence in young children and elderly
Other Invasive Infections:
- Endocarditis (heart valve infection)
- Peritonitis (abdominal infection)
- Septic arthritis (joint infection)
- Osteomyelitis (bone infection)
- Cellulitis and soft tissue infections
- Rare but serious manifestations of pneumococcal disease
Risk Factors for Pneumococcal Disease:
Age-related:
- Children under 5 years, particularly those under 2 years
- Adults over 65 years
- Age-related changes in immune function
Underlying Medical Conditions:
- Chronic heart, lung, liver, or kidney disease
- Diabetes mellitus
- Asplenia (functional or anatomic)
- Immunocompromising conditions (HIV, malignancy, transplantation)
- Cochlear implants
- Cerebrospinal fluid leaks
- Sickle cell disease and other hemoglobinopathies
Environmental and Behavioral:
- Smoking and exposure to secondhand smoke
- Alcohol abuse
- Crowded living conditions
- Daycare attendance (for children)
- Recent viral respiratory infection
- Previous antibiotic use
Demographic:
- Certain racial/ethnic groups (Alaska Native, African American, American Indian)
- Socioeconomic factors affecting access to healthcare
Public Health Impact:
Global Burden:
- Estimated 1.6 million deaths annually worldwide
- Leading cause of vaccine-preventable deaths in children under 5
- Significant economic burden through healthcare costs and productivity loss
- Disproportionate impact in low and middle-income countries
Antibiotic Resistance:
- Increasing prevalence of penicillin-resistant strains
- Multi-drug resistant pneumococci limiting treatment options
- Geographic variations in resistance patterns
- Resistance driven by inappropriate antibiotic use
Vaccination Impact:
- Significant reduction in vaccine-type invasive disease
- Herd immunity protecting unvaccinated individuals
- Serotype replacement with non-vaccine types
- Continued evolution of vaccination strategies
Special Populations:
Children:
- Higher rates of colonization and transmission
- Otitis media particularly common
- Immature immune system increases susceptibility
- Daycare attendance increases exposure risk
Elderly:
- Immunosenescence increases susceptibility
- Higher mortality rates from invasive disease
- Often present with atypical symptoms
- Comorbidities complicate management
Immunocompromised:
- Higher risk of invasive and recurrent disease
- Reduced response to vaccination
- May require additional preventive strategies
- Higher mortality despite appropriate treatment
Long-term Consequences:
Post-pneumococcal Sequelae:
- Neurological deficits following meningitis
- Hearing loss after recurrent otitis media
- Bronchiectasis following severe pneumonia
- Chronic lung disease exacerbations
- Cardiovascular complications after pneumonia
Economic and Social Impact:
- Healthcare costs for acute treatment and long-term care
- Lost productivity and educational opportunities
- Caregiver burden for those with long-term sequelae
- Quality of life impairment for survivors
The health (Content truncated due to size limit. Use line ranges to read in chunks)
