Enterococcus faecium
Overview
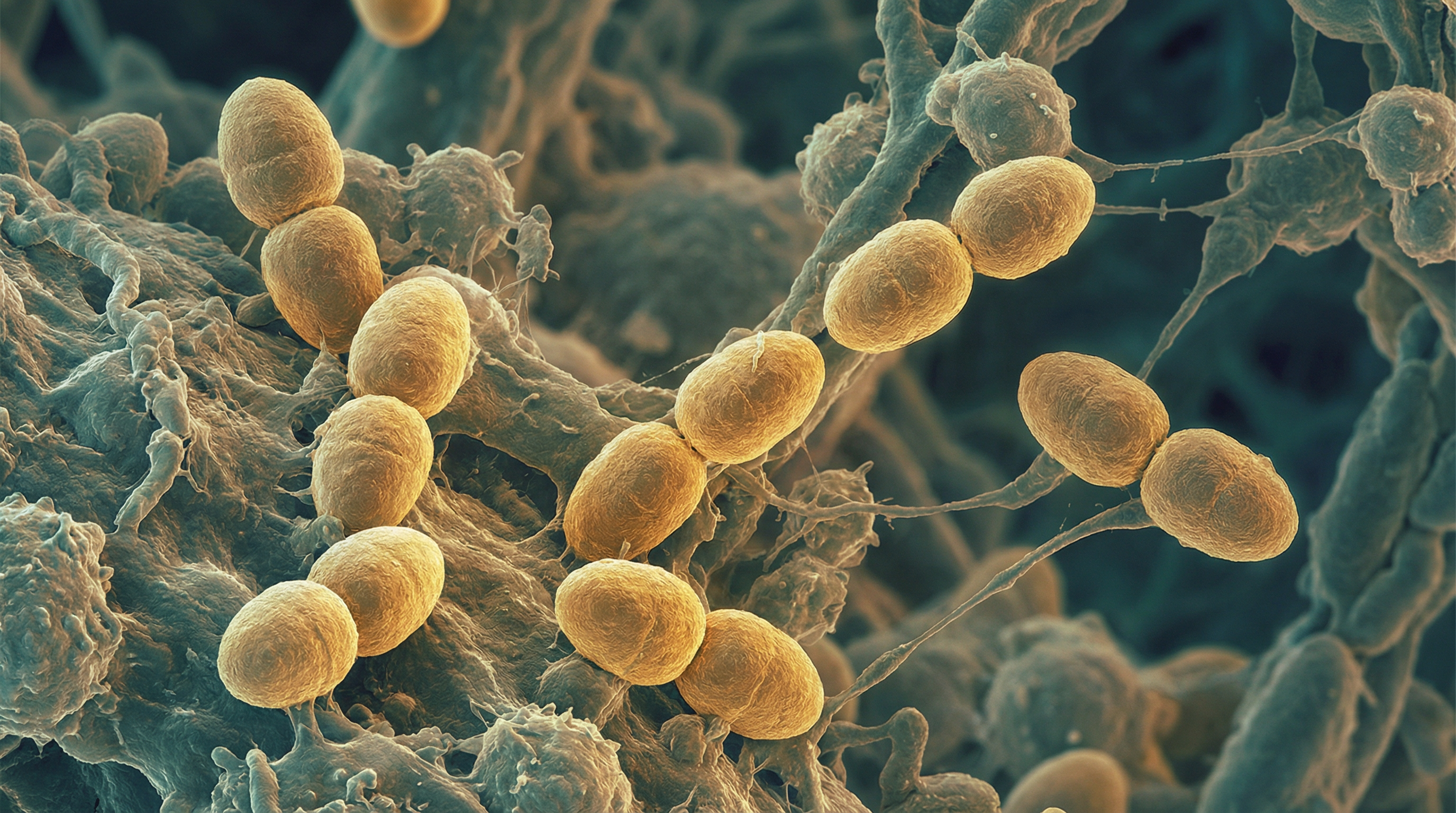
Enterococcus faecium is a Gram-positive, facultative anaerobic bacterium that has emerged as one of the most challenging nosocomial pathogens worldwide. While representing a minor component of the healthy gut microbiota, hospital-adapted E. faecium clones exhibit extraordinarily high rates of antibiotic resistance—with vancomycin resistance exceeding 80% in many regions and ampicillin resistance surpassing 70%.
Uniquely among enterococci, E. faecium has evolved distinct genetic clades with dramatically different ecological niches: hospital-associated strains (Clade A1) are genetically distinct from community commensal strains (Clade B), representing a true example of pathogen specialization.
Key Characteristics
E. faecium appears as oval-shaped diplococci or short chains. Like other enterococci, it demonstrates remarkable environmental hardiness:
- Temperature tolerance: 10-45°C (optimum 35°C)
- pH tolerance: 4.4-9.6
- Salt tolerance: Up to 6.5% NaCl
- Environmental persistence: Up to 4 months on surfaces
Clade Structure
| Clade | Ecological Niche | Genome Size | Key Features |
|---|---|---|---|
| A1 | Hospital-associated | 2,843 ± 159 genes | High resistance, ampicillin-resistant, amino sugar metabolism |
| A2 | Animal-associated | 2,597 ± 153 genes | Intermediate characteristics |
| B | Human commensal/probiotic | 2,718 ± 120 genes | Lower resistance, complex carbohydrate metabolism |
Average nucleotide identity (ANI) between Clade A and B is only 93.9-96%, suggesting near-species-level divergence.
Role in Human Microbiome
Normal Abundance
E. faecium is present at lower levels than E. faecalis in healthy individuals:
- Population density: 10⁴-10⁵ CFU/g feces (vs 10⁵-10⁷ for E. faecalis)
- Proportion: <1% of total microbiota
- Dysbiosis expansion: Can reach >30% relative abundance or 10⁹ CFU/g after antibiotics
Colonization Resistance
Obligate anaerobes directly inhibit E. faecium growth:
- Barnsiella species
- Clostridium cluster XIVa
- Blautia producta
- Bacteroides sartorii (protects via β-lactamase production)
Antibiotic disruption of these protective commensals enables VRE expansion.
Vancomycin-Resistant Enterococci (VRE)
Mechanism of Resistance
VRE resistance occurs through modification of the peptidoglycan precursor terminus:
- Normal target: D-Ala-D-Ala (vancomycin binding site)
- Modified target: D-Ala-D-Lac or D-Ala-D-Ser
- Affinity reduction: 1,000-fold decrease in vancomycin binding
vanA vs vanB Genotypes
| Feature | vanA | vanB |
|---|---|---|
| Resistance level | High (MIC >256 μg/mL) | Variable (MIC 4 to >256 μg/mL) |
| Mechanism | D-Ala-D-Lac substitution | D-Ala-D-Lac substitution |
| Teicoplanin resistance | Yes (via vanZ gene) | No |
| Genetic element | Plasmid-borne (Tn1546) | Plasmid-borne (often Tn5382) |
| Transferability | High | Inducible |
van Operon Components
- Regulatory: vanS (sensor kinase), vanR (response regulator)
- Enzymatic: vanH (dehydrogenase), vanA/B (ligase), vanX (dipeptidase)
- Modulator: vanZ (teicoplanin resistance)
Resistance Rates
| Region/Setting | VRE Rate |
|---|---|
| US E. faecium overall (2018-2019) | 62.8% |
| US CLABSI E. faecium (2011-2014) | 83.8% |
| US CAUTI E. faecium (2011-2014) | 86.2% |
| US ICU (1989) | 0.3% |
| US ICU (1999) | 25.2% |
| Australia E. faecium | >40% |
| Europe (many regions) | >40% |
Clinical Impact of VRE
| Outcome | Statistic |
|---|---|
| All-cause mortality (VRE BSI) | 32.7-33.5% |
| Attributable mortality | Up to 37% |
| Mortality fold-increase vs VSE | 2.5× |
| Annual US deaths | ~1,300 |
| Hospital stay increase | 5-12 days |
| Cost (VRE infection) | €57,675 |
| Cost (VSE infection) | €38,344 |
| Cost per patient (US) | $17,143-36,380 |
Virulence Factors
E. faecium generally exhibits fewer virulence factors than E. faecalis but compensates with higher antibiotic resistance:
| Factor | Gene | Prevalence | Function |
|---|---|---|---|
| Enterococcal surface protein | esp | 68.4% | Biofilm formation, hospital-associated |
| Cell wall adhesin | efaAfm | 46.2-100% | Tissue adherence |
| Gelatinase | gelE | 10.5-27.8% (gene); 5.3-20% (phenotype) | Tissue invasion |
| Aggregation substance | agg | 5.6-11.1% | Conjugation, adherence |
| Biofilm formation | - | 10.5% | Device colonization |
Hospital-Associated esp
E. faecium Esp is located on a pathogenicity island strongly associated with Clade A1 hospital strains. Expression is higher at 37°C and under anaerobiosis—conditions mimicking the infected host.
Clinical Infections
Epidemiology
- HAI ranking: 2nd most common pathogen in US and Europe
- BSI proportion: 38.1% of enterococcal bloodstream infections
- Primary sources: Abdominal/biliary tract (more common than E. faecalis)
- Polymicrobial rate: 28.8%
Infection Types
| Infection | E. faecium Ranking |
|---|---|
| CLABSI | 5th most frequent |
| CAUTI | 11th most frequent |
| Surgical site | 2nd most common |
| Endocarditis | Uncommon but severe |
Risk Factors for E. faecium (vs E. faecalis)
- Promoting factors: Penicillin exposure (aOR 1.99), carbapenem exposure (aOR 2.35), biliary tract source (aOR 3.36), hospital acquisition (aOR 2.58)
- Protective factors: Urinary tract source (aOR 0.49), congestive heart failure (aOR 0.51)
Mortality
| Setting | Rate |
|---|---|
| In-hospital (monomicrobial) | 21.5% |
| VRE BSI | 32.7-33.5% |
| Independent predictors | SOFA score (aOR 1.34) |
Treatment of VRE Infections
First-Line Options
| Drug | Dose | Mechanism | Notes |
|---|---|---|---|
| Daptomycin | 10-12 mg/kg IV daily | Cell membrane depolarization | Bactericidal; preferred for BSI |
| Linezolid | 600 mg PO/IV q12h | 50S ribosomal inhibition | Bacteriostatic; 85.3% microbiological cure |
Alternative Options
| Drug | Indication | Notes |
|---|---|---|
| Teicoplanin | vanB phenotypes only | Not for vanA |
| Tigecycline | Intra-abdominal infections | Not for bacteremia (low serum levels) |
| Quinupristin-dalfopristin | E. faecium only | No activity vs E. faecalis |
| Fosfomycin | UTIs | 3g PO single dose |
| Nitrofurantoin | UTIs | 100mg PO BID |
Endocarditis (VRE)
- Linezolid 600 mg IV/PO q12h for >6 weeks
- High-dose daptomycin 10-12 mg/kg with combinations (β-lactams, fosfomycin, or tigecycline)
- Relapse rate: 7-10%
Probiotic Strains: E. faecium SF68
Clinical Evidence
E. faecium SF68 (NCIMB 10415, marketed as Bioflorin) is a Clade B commensal strain with demonstrated clinical efficacy:
Acute Diarrhea Treatment (n=1,143 double-blind RCT):
- Diarrhea duration: SF68 1.69 days vs placebo 2.81 days (p<0.001)
- Day 3 outcomes: 8% with diarrhea vs 66% placebo (p<0.01)
- Pathogen clearance: Salmonella, Campylobacter, Yersinia undetectable post-treatment
Antibiotic-Associated Diarrhea Prevention (n=1,397 RCT):
- SF68 diarrhea rate: 8.6% vs placebo 16.2% (p<0.001)
- Alternative study: 8.3% vs 27.2% (p<0.01)
Adverse Events:
- RCT incidence: 1.1-1.4%
- Open-label incidence: 4.7-7.4%
Safety Considerations
Despite SF68's clinical efficacy, enterococcal probiotics carry inherent concerns:
Risks:
- Multiple virulence factors in enterococci
- Capability to translocate intestinal mucosa
- Resistance to innate immunity
- High horizontal gene transfer potential
- Leading cause of hospital-acquired infections
Safeguards:
- SF68 belongs to Clade B (commensal), distinct from Clade A1 (clinical)
- Well-characterized strain lacking 32/40 known virulence genes
- No antibiotic resistance genes in probiotic strains T110 and Symbioflor 1
- Not detectable in stools one week after cessation
Regulatory Status
- EFSA QPS status: Not included (enterococci don't meet Qualified Presumption of Safety)
- FDA: No GRAS status for any Enterococcus strain
- Licensed markets: Austria, Italy, Switzerland as pharmaceutical
Other Probiotic Applications
| Strain | Condition | Outcome |
|---|---|---|
| ENCfa68 | IBS | 62.2% normalized fecal calprotectin |
| E. faecium L3 | Allergic rhinitis | Reduced corticosteroid/antihistamine use (p<0.01) |
| E. faecium L3 | Respiratory infections | 0.29 vs 0.73 ARI cases per child (p<0.05) |
| E. faecium M74 | Hyperlipidemia | LDL 3.09 vs 3.85 mmol/L (p<0.001) |
Selective Pressure and Spread
Antibiotics promoting E. faecium/VRE:
- Vancomycin
- Extended-spectrum cephalosporins
- Metronidazole
- Anti-anaerobic agents
- Carbapenems
Healthcare Transmission
- Primary vector: Hands of healthcare workers
- Hand persistence: 60 minutes
- Surface persistence: Up to 4 months
- ICU colonization rate: 33%
- Colonized-to-infected ratio: 10:1
Metabolic Specialization
Hospital-adapted Clade A1 strains show distinct metabolic preferences:
- Utilizes: Amino sugars (N-acetylglucosamine)
- Reduced use of: Complex carbohydrates
- Adaptation: Better suited to hospital diet/environment
This metabolic shift represents a key adaptation distinguishing hospital from community strains.
Clinical Outcomes
Prognostic Factors
Good Prognosis:
- Appropriate early therapy
- Lower SOFA score
- Urinary or biliary source
- Fewer comorbidities
Poor Prognosis:
- High SOFA score
- VRE infection
- Inappropriate initial therapy
- Multiple comorbidities
- Immunocompromised state
Impact of Interventions
- Infectious disease consultation improves outcomes
- Care bundles reduce 30-day mortality from 32% to 20%
- Early microbiology alerts improve time to appropriate therapy
- Source control (device removal) often required for cure
Future Directions
E. faecium represents a critical target for:
- New antimicrobial development (limited treatment options for VRE)
- Infection control strategies in healthcare settings
- Understanding pathogen evolution and hospital adaptation
- Probiotic development with enhanced safety profiling
- Microbiome-based therapies to restore colonization resistance
- Vaccine development against key surface antigens
The dual nature of E. faecium—as both a potentially beneficial commensal and a formidable hospital pathogen—underscores the importance of strain-level characterization and the need for targeted interventions based on clade identification.
