Methanosphaera stadtmanae
Overview
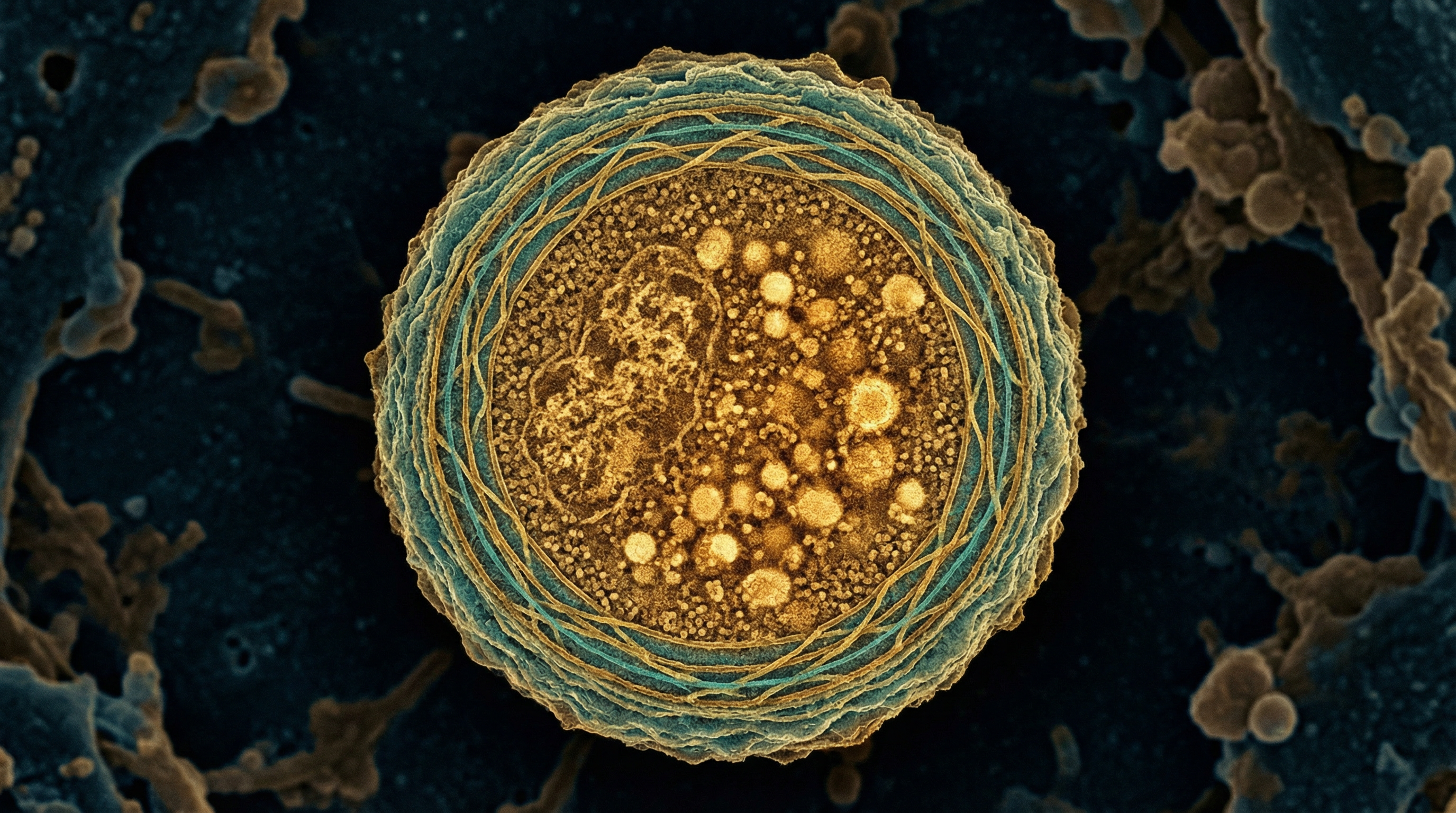
Methanosphaera stadtmanae is a methanogenic archaeon that represents the second most abundant archaeal species in the human gut microbiome, with a prevalence of approximately 20% in healthy individuals. Unlike the more common Methanobrevibacter smithii, M. stadtmanae exhibits a highly restricted energy metabolism, generating methane exclusively through the reduction of methanol with hydrogen. This specialized metabolic niche reflects its adaptation to the human intestinal environment, where it contributes to the overall microbial ecology and potentially influences host health. M. stadtmanae appears as irregular cocci organized in single cells, pairs, or most commonly tetrads, and belongs to the phylum Euryarchaeota, class Methanobacteria. Despite its relatively lower abundance compared to M. smithii, M. stadtmanae has garnered significant scientific interest due to its strong immunogenic potential, particularly its ability to induce pro-inflammatory responses through the activation of specific pattern recognition receptors. Research has implicated M. stadtmanae in various health conditions, including inflammatory bowel disease, obesity, and lung hyperresponsiveness, highlighting its potential role in host-microbe interactions beyond simple colonization. The organism's unique metabolic requirements, including its dependence on tungsten for optimal growth, further underscore its specialized adaptation to the human gut environment and present both challenges and opportunities for its study in laboratory settings.
Characteristics
Methanosphaera stadtmanae possesses several distinctive characteristics that define its biological identity:
- Taxonomic classification: Domain Archaea, Phylum Euryarchaeota, Class Methanobacteria, Order Methanobacteriales, Family Methanobacteriaceae, Genus Methanosphaera
- Morphology: Irregular cocci (spherical cells) that typically organize into single cells, pairs, or most commonly tetrads (groups of four)
- Cell wall structure: Contains pseudopeptidoglycan (pseudomurein) rather than peptidoglycan found in bacteria, making it resistant to many antibiotics that target bacterial cell wall synthesis
- Gram staining: Gram-positive reaction, though this is based on cell wall structure rather than the peptidoglycan layer found in bacteria
- Motility: Non-motile organism
- Oxygen requirements: Strictly anaerobic, requiring an oxygen-free environment for growth and survival
- Temperature preference: Mesophilic, growing optimally at human body temperature (37°C)
- pH preference: Grows optimally at neutral pH, similar to conditions in the human colon
- Nutritional requirements: Requires methanol and hydrogen as energy sources, acetate as a carbon source, and has a specific requirement for tungsten for optimal growth
- Growth characteristics: Slow-growing organism with a lag phase that can be significantly reduced by the addition of sodium tungstate to the culture medium
- Genome features: Possesses a relatively small genome that encodes a restricted metabolic capacity, including the formylmethanofuran dehydrogenase enzyme that contains tungsten
- Metabolic uniqueness: Has the most restricted energy metabolism of all methanogenic archaea, capable of generating methane only by reducing methanol with hydrogen
- Surface structures: Contains unique archaeal cell membrane lipids with ether-linked isoprenoid chains, distinct from bacterial fatty acid-based membranes
- Immunogenic properties: RNA serves as a microbe-associated molecular pattern (MAMP) that can be recognized by the human immune system
- Cultivation challenges: Fastidious organism that is difficult to isolate and culture, with only one strain available in public collections until recently
M. stadtmanae's distinctive characteristics reflect its specialized adaptation to the human gut environment. Its restricted metabolism, specific nutritional requirements (particularly for tungsten), and unique cell structure distinguish it from both bacteria and other archaea. These features have made it challenging to study in laboratory settings, contributing to the relatively limited understanding of its role in the human microbiome compared to more abundant bacterial species. However, its unique characteristics also make it an interesting model organism for studying archaeal-host interactions and the potential contributions of archaea to human health and disease.
Role in Human Microbiome
Methanosphaera stadtmanae occupies a specialized niche within the human microbiome:
Prevalence and distribution:
- Present in approximately 20% of healthy individuals
- Represents the second most abundant archaeal species in the human gut microbiome
- Primarily inhabits the colon and rectum
- Less abundant than Methanobrevibacter smithii, which is found in up to 95% of individuals
Ecological significance:
- Functions as a hydrogenotrophic methanogen, consuming hydrogen produced by bacterial fermentation
- Contributes to the removal of excess hydrogen from the gut ecosystem
- Helps maintain the redox balance in the gut environment
- May influence the efficiency of bacterial fermentation processes
Population dynamics:
- Often exhibits mutual exclusion with Methanobrevibacter smithii in the gut microbiota
- In some individuals, M. stadtmanae may be the dominant methanogen rather than M. smithii
- When dominant, can comprise up to 78% of the archaeal population in those individuals
- Abundance may vary based on diet, host genetics, and other environmental factors
Microbial interactions:
- Depends on fermentative bacteria that produce methanol as a substrate
- May form syntrophic relationships with specific bacterial groups
- Competes with other hydrogen-consuming microorganisms such as acetogenic bacteria and sulfate-reducing bacteria
- Potentially influences the composition and activity of the bacterial community
Host-microbe interface:
- Recognized by the host immune system through its RNA
- Interacts with pattern recognition receptors, particularly TLR7 and TLR8
- May contribute to the education and regulation of the immune system
- Potential role in maintaining gut homeostasis through these interactions
Developmental aspects:
- Acquisition pathway in humans is not fully understood
- May be transmitted vertically from mother to infant
- Colonization likely increases throughout childhood and stabilizes in adulthood
- Persistence patterns across the human lifespan remain to be fully characterized
Geographical and ethnic variations:
- Prevalence and abundance may vary across different human populations
- Influenced by dietary habits, cultural practices, and possibly host genetics
- Studies in Southeast Asian populations have shown presence but at varying levels
- Most research has focused on Western populations, creating potential knowledge gaps
M. stadtmanae's role in the human microbiome extends beyond simple colonization. Its specialized metabolism and interactions with both the microbial community and the host immune system suggest it may have important functional contributions to gut ecology and potentially host health. The observed mutual exclusion with M. smithii in many individuals raises interesting questions about niche specialization and adaptation strategies among gut methanogens. Despite being less abundant than many bacterial species, M. stadtmanae's unique metabolic activities and immunomodulatory properties make it an important component of the human gut microbiome worthy of continued research attention.
Health Implications
Methanosphaera stadtmanae has several significant implications for human health:
Immune system modulation:
- Potent stimulator of the innate immune system
- RNA serves as a microbe-associated molecular pattern (MAMP) recognized by TLR7 and TLR8
- Induces TLR8-dependent NLRP3 inflammasome activation
- Triggers production of pro-inflammatory cytokines including TNF-α and IL-1β
- Stimulates an antiviral-type immune response with type-I and type-III interferon production
- May contribute to immune education and homeostasis in healthy individuals
Inflammatory bowel disease (IBD):
- Potentially involved in the development or exacerbation of IBD
- Higher abundance observed in some IBD patients
- Pro-inflammatory properties may contribute to intestinal inflammation
- Interaction with genetic susceptibility factors may influence disease risk
- Could represent a target for therapeutic intervention in IBD
Obesity and metabolic disorders:
- Implicated in the development of obesity in some studies
- May influence energy harvest from diet through effects on microbial fermentation
- Potential impact on host metabolism through hydrogen consumption
- Complex and sometimes contradictory associations with body weight regulation
- Interactions with diet may influence metabolic outcomes
Respiratory conditions:
- Associated with lung hyperresponsiveness
- Potential role in allergic airway inflammation
- May influence lung-gut axis through systemic immune effects
- Mechanism likely involves immune activation rather than direct colonization
Cancer associations:
- Some studies suggest potential links to cancer development
- May influence inflammation-associated carcinogenesis
- Could affect the tumor microenvironment through immune modulation
- Research in this area remains preliminary and requires further investigation
Gastrointestinal function:
- Potential influence on gut motility through methane production
- May affect intestinal transit time
- Could contribute to bloating and abdominal discomfort in some individuals
- Interactions with the enteric nervous system remain to be fully characterized
Individual variation in response:
- Host genetic factors likely influence immune response to M. stadtmanae
- Variation in TLR7/8 expression or function may affect recognition
- Pre-existing inflammatory conditions may modify the impact of colonization
- Diet and lifestyle factors may modulate the health effects
Therapeutic considerations:
- Potential target for interventions in inflammatory conditions
- Manipulation of its abundance might influence disease outcomes
- Tungsten supplementation could potentially modify its activity
- Archaeal-specific antimicrobials might offer selective targeting options
The health implications of M. stadtmanae are complex and context-dependent. While its pro-inflammatory properties suggest potential negative impacts in susceptible individuals or disease states, these same immunomodulatory effects may contribute to normal immune development and function in healthy hosts. The organism's restricted prevalence (approximately 20% of individuals) compared to M. smithii (up to 95%) raises interesting questions about its evolutionary relationship with human hosts and whether its presence represents a commensal, mutualistic, or potentially pathobiotic relationship depending on context. Further research is needed to fully elucidate the mechanisms through which M. stadtmanae influences human health and to determine whether targeting this archaeon might offer therapeutic benefits in specific conditions.
Metabolic Activities
Methanosphaera stadtmanae exhibits highly specialized metabolic capabilities that distinguish it from other methanogens:
Core methanogenesis pathway:
- Utilizes a restricted form of methanogenesis
- Generates methane exclusively by reducing methanol with hydrogen: CH₃OH + H₂ → CH₄ + H₂O
- This reaction yields energy in the form of ATP through chemiosmotic coupling
- Cannot use carbon dioxide as a carbon source for methanogenesis, unlike many other methanogens
- Has the most restricted energy metabolism of all methanogenic archaea
Carbon assimilation:
- Utilizes acetate as the main carbon source for biosynthesis
- Cannot fix carbon dioxide for cell carbon
- Lacks many of the carbon fixation pathways found in other methanogens
- Depends on external sources of organic carbon compounds
Nutritional requirements:
- Absolute requirement for methanol as a methyl group donor
- Depends on hydrogen as an electron donor
- Requires acetate for biosynthetic purposes
- Enhanced growth with tungsten supplementation
- Tungsten is incorporated into the formylmethanofuran dehydrogenase enzyme
- May utilize selenium, though this appears less critical than tungsten
Enzymatic machinery:
- Possesses formylmethanofuran dehydrogenase, a tungsten-iron-sulfur protein
- Contains methanol:coenzyme M methyltransferase for methanol activation
- Has methyl-coenzyme M reductase for the final step of methane formation
- Lacks many of the enzymes required for alternative methanogenic pathways
- Genome encodes a restricted set of metabolic enzymes compared to other methanogens
Energy conservation:
- Employs a hydrogen-dependent reduction of methanol to methane as primary energy source
- This process is coupled to the generation of a proton motive force
- ATP synthesis occurs via a membrane-bound ATP synthase
- Relatively inefficient energy metabolism compared to methanogens with broader substrate range
Adaptations to gut environment:
- Metabolic specialization allows exploitation of a specific niche in the gut ecosystem
- Utilizes methanol produced by bacterial fermentation of pectin and other compounds
- Consumes hydrogen, helping to maintain favorable conditions for fermentative bacteria
- Metabolic restrictions may limit competition with other microorganisms
Growth characteristics:
- Slow growth rate due to limited energy yield from restricted metabolism
- Growth significantly enhanced by tungsten supplementation
- Addition of sodium tungstate reduces lag phase from 5-7 days to approximately 48 hours
- Exponential growth phase typically reached after 6 days in standard culture conditions
Metabolic interactions with other microbes:
- Depends on bacteria that produce methanol from pectin and other substrates
- Competes with other hydrogen-consuming microorganisms
- May influence bacterial fermentation patterns through hydrogen consumption
- Potential syntrophic relationships with specific bacterial groups
The metabolic activities of M. stadtmanae reflect its specialized adaptation to a specific niche within the human gut ecosystem. Its restricted metabolism, focused exclusively on methanol reduction with hydrogen, allows it to exploit a metabolic opportunity while avoiding competition with more versatile methanogens like M. smithii. The requirement for tungsten, which enhances its growth significantly, represents an interesting adaptation that may influence its ecological distribution and abundance. This metabolic specialization likely contributes to the observed mutual exclusion with M. smithii in many individuals, suggesting different niche adaptation strategies among human gut methanogens. Understanding these unique metabolic features provides insights into how M. stadtmanae persists in the competitive gut environment despite its restricted metabolic capabilities.
Clinical Relevance
Methanosphaera stadtmanae has several aspects of clinical significance:
Diagnostic considerations:
- Detection methods include PCR-based molecular techniques targeting 16S rRNA genes
- Cultivation requires specialized anaerobic techniques and media
- Growth enhanced by tungsten supplementation, which may improve isolation success
- Breath methane testing may indirectly indicate presence, though cannot distinguish between different methanogens
- Limited availability of reference strains complicates standardized testing
Inflammatory conditions:
- Strong immunogenic potential relevant to inflammatory disorders
- Activates TLR8-dependent NLRP3 inflammasome pathway
- Induces pro-inflammatory cytokines including TNF-α and IL-1β
- Potential role in inflammatory bowel disease pathogenesis
- May contribute to systemic inflammation in susceptible individuals
Respiratory health:
- Associated with lung hyperresponsiveness
- Potential involvement in allergic airway inflammation
- May influence lung-gut axis through immune modulation
- Could represent a target for intervention in certain respiratory conditions
- Mechanism likely involves systemic immune effects rather than direct colonization
Metabolic health:
- Complex relationship with obesity and metabolic disorders
- May influence energy harvest from diet
- Potential impact on host metabolism through effects on the gut microbiome
- Interactions with diet may modify metabolic outcomes
- Research shows sometimes contradictory associations with body weight
Therapeutic approaches:
- Potential target for interventions in inflammatory conditions
- Archaeal-specific agents might offer selective targeting
- Dietary modifications could potentially alter its abundance
- Tungsten modulation might influence its activity
- Immunomodulatory approaches targeting its recognition pathways
Biomarker potential:
- Presence or abundance may serve as a biomarker for certain conditions
- Changes in M. stadtmanae levels might indicate shifts in gut ecology
- Ratio to M. smithii could provide insights into gut microbiome status
- Immune responses to M. stadtmanae might reflect underlying inflammatory predisposition
- Longitudinal monitoring could track disease progression or treatment response
Research challenges:
- Difficult to culture using standard microbiological techniques
- Limited number of available reference strains
- Requires specialized growth conditions and media
- Slow growth rate complicates experimental studies
- Mutual exclusion with M. smithii may confound microbiome analyses
Clinical testing considerations:
- Not routinely included in standard microbiome testing panels
- Specialized PCR assays required for detection
- Quantification methods not standardized across laboratories
- Interpretation of results complicated by limited normative data
- Clinical significance of detection remains to be fully established
The clinical relevance of M. stadtmanae stems primarily from its immunomodulatory properties and potential involvement in inflammatory conditions. Its ability to activate the NLRP3 inflammasome through TLR8 represents a unique interaction between archaea and the human immune system that may have implications for various inflammatory disorders. The observed associations with conditions like inflammatory bowel disease and lung hyperresponsiveness suggest potential pathogenic roles in susceptible individuals, though causality remains to be firmly established. From a diagnostic perspective, the challenges in cultivating and detecting M. stadtmanae have limited its inclusion in routine clinical testing, though advances in molecular methods are improving this situation. As research continues to elucidate its role in human health and disease, M. stadtmanae may emerge as an important target for therapeutic interventions or a valuable biomarker for certain clinical conditions.
Interactions with Other Microorganisms
Methanosphaera stadtmanae engages in complex interactions with other members of the human microbiome:
Relationship with Methanobrevibacter smithii:
- Exhibits mutual exclusion with M. smithii in more than 80% of individuals
- When present, often dominates the archaeal community in those lacking significant M. smithii
- Represents an alternative dominant methanogen in a minority of individuals
- Comparative genomic analyses suggest different niche adaptation strategies
- This mutual exclusion pattern may reflect competition for similar resources or ecological niches
Dependence on bacterial fermentation:
- Relies on bacteria that produce methanol as a substrate
- Methanol in the gut primarily derives from bacterial fermentation of pectin
- Potential syntrophic relationships with pectin-degrading bacteria
- May influence the composition and activity of these bacterial groups
- Ecological success depends on the presence of these bacterial partners
Hydrogen economy interactions:
- Competes with other hydrogen-consuming microorganisms
- Alternative hydrogen consumers include acetogenic bacteria and sulfate-reducing bacteria
- Competition for hydrogen may influence community structure and function
- Hydrogen utilization affects the thermodynamics of bacterial fermentation
- May indirectly influence bacterial metabolism through hydrogen removal
Impact on bacterial metabolism:
- Consumption of hydrogen can shift bacterial fermentation toward more oxidized end products
- May enhance the efficiency of bacterial energy harvest
- Could influence the production of short-chain fatty acids
- Potential effects on bacterial gene expression and metabolic regulation
- These interactions contribute to overall ecosystem function
Biofilm participation:
- May contribute to multispecies biofilm formation in the gut
- Biofilm association could enhance persistence and stability
- Potential spatial organization within microbial communities
- Biofilms may provide protection against host defenses
- Could facilitate metabolic exchange with bacterial partners
Immune-mediated interactions:
- Immune responses triggered by M. stadtmanae may affect other microorganisms
- Inflammation could alter the gut environment, influencing community composition
- Pro-inflammatory effects might disrupt mucosal barrier function
- These indirect effects could reshape the microbiome more broadly
- May contribute to dysbiosis in inflammatory conditions
Co-occurrence patterns:
- Shows specific patterns of co-occurrence with certain bacterial taxa
- These patterns may reflect complementary metabolic capabilities
- Understanding these patterns helps elucidate community assembly rules
- May indicate metabolic networks or functional guilds within the microbiome
- Could provide insights into ecological succession and stability
Adaptation to specific host factors:
- Different distribution compared to M. smithii may reflect adaptation to host-specific factors
- Potential influence of host genetics, diet, and immune status on these interactions
- May occupy different microenvironments within the gut ecosystem
- Temporal dynamics could vary in response to changing conditions
- These adaptations contribute to the complexity of the gut microbiome
The interactions between M. stadtmanae and other microorganisms in the human gut represent a fascinating aspect of microbiome ecology. The observed mutual exclusion with M. smithii is particularly intriguing, suggesting distinct ecological strategies among human gut methanogens despite their phylogenetic relatedness. This pattern may reflect competition for similar resources, different responses to host factors, or complex ecological dynamics that remain to be fully elucidated. The dependence of M. stadtmanae on bacterial partners for methanol production highlights the interconnected nature of the gut ecosystem, where archaeal metabolism is intimately linked to bacterial activities. These interactions contribute to the overall function of the microbiome and may have important implications for host health, particularly in the context of inflammatory conditions where M. stadtmanae's immunomodulatory properties could influence community dynamics.
Research Significance
Methanosphaera stadtmanae has substantial significance across multiple research domains:
Archaeal biology and evolution:
- Represents one of the few archaea consistently found in the human body
- Provides insights into archaeal adaptation to the mammalian gut environment
- Illustrates specialized metabolic evolution with extremely restricted energy metabolism
- Demonstrates how archaea can occupy specific niches within complex microbial communities
- Contributes to understanding the evolutionary history of host-associated archaea
Microbiome science:
- Highlights the importance of considering archaea in human microbiome studies
- Demonstrates mutual exclusion patterns that inform community assembly rules
- Provides evidence for niche specialization among human gut methanogens
- Contributes to understanding the "hydrogen economy" of the gut ecosystem
- Illustrates how less abundant members can have significant functional impacts
Immunology:
- First archaeon shown to be specifically recognized by human pattern recognition receptors
- Revealed archaeal RNA as a microbe-associated molecular pattern (MAMP)
- Demonstrated TLR8-dependent NLRP3 inflammasome activation by an archaeon
- Provides insights into how the immune system interacts with non-bacterial microbes
- Contributes to understanding the immunomodulatory potential of the microbiome
Metabolic research:
- Exemplifies extreme metabolic specialization (methanol reduction with hydrogen)
- Illustrates the importance of trace elements (tungsten) in microbial metabolism
- Provides a model for studying hydrogen utilization in the gut
- Contributes to understanding methanogenesis in human-associated environments
- May inform research on microbial contributions to host metabolism
Inflammatory disease mechanisms:
- Potential role in inflammatory bowel disease pathogenesis
- Associations with lung hyperresponsiveness suggest gut-lung axis involvement
- Provides mechanistic insights into microbiome contributions to inflammation
- May help explain individual variation in inflammatory disease susceptibility
- Could lead to new therapeutic targets for inflammatory conditions
Methodological advances:
- Drives development of techniques for culturing fastidious anaerobic archaea
- Tungsten supplementation finding may improve isolation of other archaea
- Encourages inclusion of archaeal-specific methods in microbiome analysis
- Highlights the importance of multi-omics approaches in microbiome research
- Advances in studying M. stadtmanae have broader applications in archaeal biology
Therapeutic development:
- Identified as a potential therapeutic target for inflammatory conditions
- Offers opportunity for domain-specific antimicrobial approaches
- Understanding its immunomodulatory properties may lead to novel anti-inflammatory strategies
- Manipulation of its abundance or activity could influence disease outcomes
- May contribute to precision medicine approaches based on microbiome composition
Ecological modeling:
- Mutual exclusion with M. smithii provides a model for studying competitive dynamics
- Helps explain community assembly and stability in the gut microbiome
- Contributes to understanding how specialized metabolic niches evolve
- Informs ecological theories about diversity maintenance in microbial communities
- May improve predictive modeling of microbiome responses to perturbations
The research significance of M. stadtmanae extends far beyond its specific role in the human gut, touching on fundamental questions in microbiology, immunology, ecology, and medicine. As one of the few archaeal species consistently found in humans, it serves as an important model organism for understanding the contributions of this often-overlooked domain of life to human health and disease. The discovery of its immunomodulatory properties and the molecular mechanisms of its recognition by the human immune system represent major advances in our understanding of host-microbe interactions. Continued research on this organism promises to yield insights with broad applications in both basic science and clinical medicine, particularly in the context of inflammatory disorders where its unique properties may offer new therapeutic opportunities.
