Streptococcus mitis
Streptococcus mitis is a gram-positive, facultatively anaerobic coccus that is a prominent member of the human oral microbiome. As part of the viridans group streptococci (VGS), S. mitis primarily colonizes the oral cavity and upper respiratory tract, where it typically exists as a commensal organism. However, it can also act as an opportunistic pathogen in certain circumstances, particularly in immunocompromised individuals.
Key Characteristics
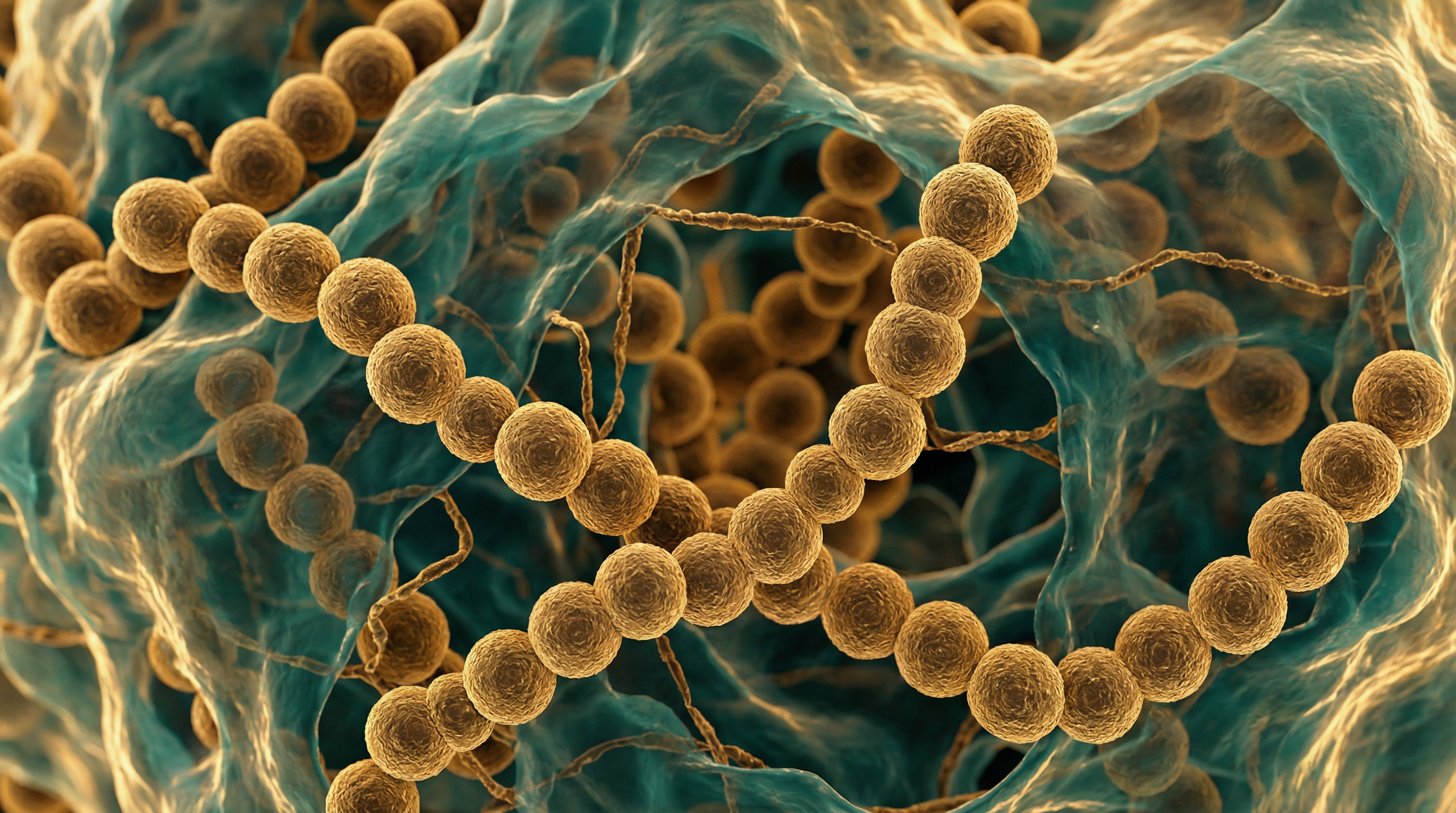
S. mitis belongs to the mitis group of streptococci, which includes closely related species such as S. pneumoniae, S. oralis, and S. infantis. It appears as chains of cocci under microscopic examination and forms alpha-hemolytic colonies on blood agar. The bacterium is non-motile, catalase-negative, and optochin-resistant (unlike S. pneumoniae, which is optochin-sensitive).
S. mitis is biochemically inert compared to many other streptococci, which can make species-level identification challenging in clinical laboratories. It typically does not ferment inulin, mannitol, or sorbitol, and does not hydrolyze esculin. The bacterium possesses various surface proteins that facilitate adhesion to host tissues and interactions with other microorganisms.
Genomic analysis has revealed significant genetic diversity within the S. mitis group, with evidence suggesting that S. mitis may actually comprise multiple distinct species. This genetic heterogeneity contributes to the variable phenotypic characteristics and pathogenic potential observed among different S. mitis strains.
Role in Human Microbiome
S. mitis is one of the most common and abundant bacteria in the human oral cavity. It is found primarily on:
- Buccal mucosa
- Tongue dorsum
- Dental plaque
- Saliva
- Oropharynx
As an early colonizer of oral surfaces, S. mitis contributes to the development and maturation of the oral microbiome. It is among the first bacteria to colonize the oral cavity of newborns and remains a stable component of the oral microbiome throughout life. The bacterium plays a role in establishing the ecological balance of the oral microbiome by:
- Occupying niches that might otherwise be colonized by pathogenic bacteria
- Participating in biofilm formation and interspecies communication
- Modulating local immune responses
- Competing with potential pathogens for nutrients and attachment sites
S. mitis is considered part of the core oral microbiome and is generally associated with oral health when present in appropriate abundance and in balance with other commensal species.
Health Implications
Commensal Role
As a commensal organism, S. mitis contributes to oral health through several mechanisms:
Colonization resistance: By occupying niches in the oral cavity, S. mitis can prevent colonization by more pathogenic species.
Immune modulation: S. mitis can activate the aryl hydrocarbon receptor (AhR) in oral epithelial cells, which plays a role in regulating local immune responses and maintaining mucosal homeostasis.
Cross-protective immunity: Antigenic similarities between S. mitis and S. pneumoniae may contribute to the development of natural immunity against pneumococcal infections.
Biofilm regulation: S. mitis participates in the formation of balanced, health-associated oral biofilms.
Pathogenic Potential
Despite its predominant role as a commensal, S. mitis can act as an opportunistic pathogen under certain conditions:
Infective endocarditis: S. mitis is among the viridans streptococci most frequently isolated from cases of infective endocarditis, particularly in patients with pre-existing heart valve abnormalities.
Bacteremia: In immunocompromised patients, especially those with cancer and neutropenia, S. mitis can cause bacteremia that may range from asymptomatic to severe, including a condition known as "viridans streptococcal shock syndrome."
Respiratory infections: S. mitis has been implicated in pneumonia and other respiratory infections, particularly in hospitalized or immunocompromised patients.
Meningitis: Rarely, S. mitis can cause meningitis, especially following neurosurgical procedures or in immunocompromised individuals.
Research has shown that certain S. mitis strains are more likely to cause severe clinical disease than others, suggesting strain-specific virulence factors that may contribute to pathogenicity.
Metabolic Activities
S. mitis exhibits various metabolic capabilities that enable it to thrive in the oral environment:
Carbohydrate metabolism: It can ferment various sugars, including glucose, sucrose, and lactose, producing primarily lactic acid as an end product.
Arginine metabolism: Some strains can metabolize arginine via the arginine deiminase system, which can help neutralize acidic conditions in the oral cavity.
Hydrogen peroxide production: S. mitis produces hydrogen peroxide, which can inhibit the growth of competing bacteria but may also contribute to oxidative stress in host tissues.
Extracellular polysaccharide synthesis: It can produce extracellular polysaccharides that contribute to biofilm formation and adhesion to oral surfaces.
Neuraminidase activity: Some strains produce neuraminidase enzymes that can cleave sialic acid residues from host glycoproteins, potentially facilitating adhesion and nutrient acquisition.
These metabolic activities allow S. mitis to adapt to the dynamic conditions of the oral environment and contribute to its ecological success as a commensal organism.
Clinical Relevance
The clinical significance of S. mitis primarily relates to its role as an opportunistic pathogen:
Infective endocarditis: S. mitis is a common cause of viridans streptococcal endocarditis, which typically has a subacute presentation. Antibiotic prophylaxis may be recommended for high-risk patients undergoing dental procedures.
Bacteremia in cancer patients: S. mitis bacteremia in neutropenic cancer patients can range from asymptomatic to severe, with some patients developing viridans streptococcal shock syndrome characterized by hypotension, acute respiratory distress syndrome, and multi-organ failure.
Antibiotic resistance: Increasing resistance to beta-lactams, macrolides, and other antibiotics has been observed in S. mitis isolates, complicating treatment of infections.
Diagnostic challenges: Accurate identification of S. mitis in clinical specimens can be challenging due to its phenotypic similarities to other viridans streptococci and S. pneumoniae.
Biomarker potential: The presence or abundance of S. mitis in oral samples is being investigated as a potential biomarker for various oral and systemic conditions.
Understanding the factors that determine whether S. mitis acts as a commensal or pathogen in different host contexts remains an important area of clinical research.
Interaction with Other Microorganisms
S. mitis engages in complex interactions with other members of the oral microbiome:
Coaggregation: It can coaggregate with various oral bacteria, including Actinomyces species and other streptococci, contributing to biofilm formation.
Interspecies communication: S. mitis participates in quorum sensing and may influence the behavior of other bacteria through signaling molecules.
Competition with pathogens: Through hydrogen peroxide production and competition for nutrients, S. mitis can inhibit the growth of potential pathogens like Streptococcus mutans.
Synergistic relationships: In some contexts, S. mitis may form synergistic relationships with other bacteria, potentially enhancing biofilm formation or virulence.
Horizontal gene transfer: S. mitis can exchange genetic material with other streptococci, including virulence factors and antibiotic resistance genes. This is particularly significant with S. pneumoniae, with which it shares extensive genomic similarities.
These interactions contribute to the ecological balance of the oral microbiome and can influence both health and disease states.
Research Significance
S. mitis has become an important focus of research for several reasons:
Evolutionary relationships: As a close relative of S. pneumoniae, studying S. mitis provides insights into the evolution of pathogenicity within the streptococci.
Commensal-pathogen transition: Understanding the factors that determine whether S. mitis acts as a commensal or pathogen may reveal general principles about opportunistic infections.
Antibiotic resistance: S. mitis can serve as a reservoir for antibiotic resistance genes that may be transferred to more pathogenic species.
Vaccine development: The antigenic similarities between S. mitis and S. pneumoniae have implications for pneumococcal vaccine design and efficacy.
Cancer microbiome: Recent research has investigated the role of S. mitis in the microbiome of cancer patients and its potential influence on treatment outcomes.
Oral-systemic health connections: As a prominent member of the oral microbiome, S. mitis may play a role in the connections between oral health and systemic conditions.
Continued research on S. mitis promises to enhance our understanding of host-microbe interactions in both health and disease contexts.
References
Shelburne SA, Sahasrabhojane P, Saldana M, et al. Streptococcus mitis strains causing severe clinical disease in cancer patients. Emerg Infect Dis. 2014;20(5):762-771.
Mitchell J. Streptococcus mitis: walking the line between commensalism and pathogenesis. Mol Oral Microbiol. 2011;26(2):89-98.
Denapaite D, Brückner R, Nuhn M, et al. The genome of Streptococcus mitis B6--what is a commensal? PLoS One. 2010;5(2):e9426.
Kilian M, Riley DR, Jensen A, Brüggemann H, Tettelin H. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. mBio. 2014;5(4):e01490-14.
Do T, Jolley KA, Maiden MC, et al. Population structure of Streptococcus oralis. Microbiology (Reading). 2009;155(Pt 8):2593-2602.
Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92(6):485-491.
