Malassezia restricta
Overview
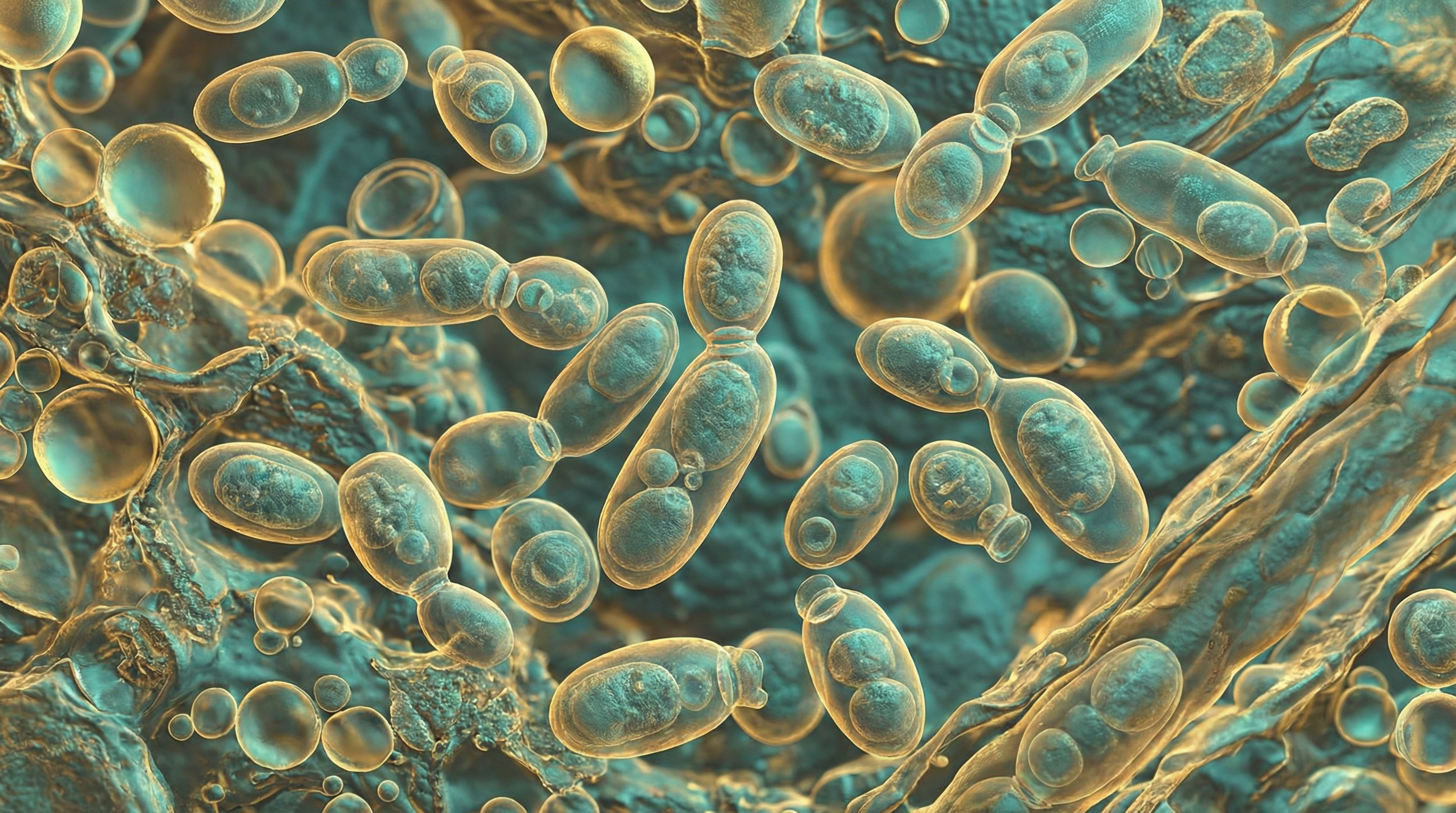
Malassezia restricta is a lipid-dependent, basidiomycetous yeast that stands as one of the most prevalent fungal species on human skin. As a dominant member of the human skin mycobiome, M. restricta is found on virtually all individuals from birth and persists throughout life. This ubiquitous fungus primarily colonizes sebaceous-rich areas of the skin, including the scalp, face, and upper trunk, where it can access the lipids essential for its growth and survival.
M. restricta belongs to the genus Malassezia, which currently comprises 18 recognized species, with M. restricta and M. globosa being the two most abundant species on human skin. The genus Malassezia has undergone several taxonomic reclassifications throughout history. Originally discovered in the late 19th century by Malassez and Sabouraud, these fungi were difficult to cultivate, leading to confusion about their classification. M. restricta was formally described as a distinct species in 2000, following molecular phylogenetic analyses that helped clarify the taxonomy of the Malassezia genus.
What makes M. restricta particularly interesting from a biological perspective is its obligate lipid dependency. Through evolutionary adaptation, M. restricta has lost the genes necessary for de novo fatty acid synthesis, making it entirely reliant on exogenous lipids from its host. This adaptation has allowed M. restricta to specialize in colonizing the lipid-rich microenvironments of human skin, where it can access the sebaceous secretions it requires for growth.
Despite its abundance on human skin, M. restricta has been historically challenging to study due to its fastidious growth requirements and difficulty in laboratory cultivation. It grows poorly in standard culture media, requiring specialized lipid-supplemented media for isolation and propagation. Additionally, its thick cell wall makes it resistant to lysis, creating challenges for molecular analyses. These technical difficulties have historically limited our understanding of this organism, though recent advances in genomic and metagenomic technologies have begun to shed light on its biology and ecological role.
M. restricta exists primarily as a commensal organism, living in harmony with its human host without causing disease. However, under certain conditions, it can transition to an opportunistic pathogen, contributing to common skin disorders such as dandruff, seborrheic dermatitis, atopic dermatitis, and psoriasis. The factors that trigger this transition from commensal to pathogen are not fully understood but likely involve changes in host immunity, skin barrier function, sebum composition, and interactions with other members of the skin microbiome.
The relationship between M. restricta and human health represents a complex interplay between fungal factors, host characteristics, and environmental influences. Understanding this relationship is crucial for developing effective strategies to maintain skin health and treat Malassezia-associated skin disorders, which affect millions of people worldwide.
Characteristics
Malassezia restricta possesses several distinctive characteristics that define its biology and ecological niche on human skin:
Morphological Features:
- Small oval to globose yeast cells, typically 2-4 μm in diameter
- Reproduces asexually through unipolar budding, forming small buds with a narrow base
- Does not form pseudohyphae or true hyphae under normal conditions
- Lacks the ability to form filaments, unlike some other Malassezia species
- Colonies on specialized media appear cream to yellowish, with a smooth, glistening surface
- Growth is typically slow compared to other yeasts, requiring 5-7 days for visible colony formation
Cell Wall Structure:
- Possesses an exceptionally thick cell wall (up to 0.25 μm) compared to other yeasts
- Cell wall is rich in lipids (15-20% of dry weight), contributing to its hydrophobic surface
- Contains unique cell wall mannans and β-glucans that interact with host immune receptors
- The thick, lipid-rich cell wall provides resistance to environmental stressors and host defenses
- Cell wall structure makes it resistant to standard cell lysis procedures, complicating molecular analyses
- Surface is decorated with various adhesins that facilitate attachment to host surfaces
Genomic Features:
- Compact genome of approximately 7.2 Mb, one of the smallest among fungi
- Haploid genome with approximately 4,000 protein-coding genes
- Has undergone significant gene loss, particularly genes involved in carbohydrate metabolism and fatty acid synthesis
- Contains expanded families of genes encoding lipases, phospholipases, and proteases
- Possesses multiple copies of genes involved in lipid acquisition and metabolism
- Genomic evidence of adaptation to the skin environment through retention of genes for stress tolerance and immune evasion
Metabolic Capabilities:
- Obligate lipid dependency due to inability to synthesize fatty acids de novo
- Requires external sources of long-chain fatty acids (C14 or longer) for growth
- Possesses multiple lipases and phospholipases to harvest lipids from the environment
- Limited carbohydrate utilization capabilities compared to other fungi
- Capable of metabolizing sebaceous lipids into various bioactive compounds
- Produces oxylipins that can modulate host immune responses
- Metabolizes squalene and other skin lipids, potentially generating irritant byproducts
Growth Requirements:
- Optimal growth temperature of 32-35°C, corresponding to human skin temperature
- Prefers slightly acidic pH (5.5-6.0), matching the natural pH of human skin
- Requires specialized media supplemented with lipids (e.g., olive oil, Tween compounds)
- Growth is enhanced by the addition of glycine and thiamine to culture media
- Tolerant of the high salt concentrations found on human skin
- Capable of growth under relatively low oxygen conditions, similar to those in hair follicles
- Extremely difficult to cultivate in laboratory settings compared to other skin fungi
Ecological Adaptations:
- Highly adapted to the sebaceous microenvironment of human skin
- Preferentially colonizes lipid-rich areas such as scalp, face, and upper trunk
- Present in both sebaceous and non-sebaceous skin sites, though more abundant in the former
- Forms biofilms on skin surfaces, enhancing persistence and resistance
- Capable of adhering to corneocytes (dead skin cells) through specific adhesins
- Population density fluctuates with changes in sebum production throughout life
- Demonstrates remarkable host specificity, with strains adapted to human skin
Virulence Factors:
- Produces multiple extracellular hydrolytic enzymes, including lipases, phospholipases, and proteases
- Secretes lipases that can degrade sebum triglycerides, releasing potentially irritating free fatty acids
- Generates lipid peroxides through metabolism of skin lipids, which may damage skin cells
- Possesses mechanisms to evade host immune responses, including complement inhibition
- Capable of inducing inflammatory cytokine production by keratinocytes
- Produces indole compounds with immunomodulatory properties
- Forms biofilms that enhance resistance to host defenses and antifungal agents
Genetic Diversity:
- Exhibits considerable strain-to-strain variation in virulence potential
- Different strains show varying enzyme production profiles
- Genomic evidence suggests possible recombination despite no observed sexual cycle
- Strain diversity may explain variable clinical presentations of Malassezia-associated conditions
- Population structure appears to be primarily clonal with limited geographic clustering
- Some strains show enhanced ability to induce inflammatory responses
- Genetic diversity likely contributes to adaptation to different host microenvironments
These characteristics collectively enable M. restricta to thrive as a dominant member of the human skin mycobiome. Its specialized adaptations for lipid acquisition and metabolism, coupled with mechanisms to persist despite host defenses, allow it to maintain a stable presence on human skin throughout life. The balance between its commensal nature and pathogenic potential is influenced by these intrinsic characteristics, along with host factors and environmental conditions.
Role in Human Microbiome
Malassezia restricta occupies a central position in the human skin microbiome, particularly within the mycobiome (fungal component), where it plays multiple ecological roles:
Distribution and Abundance:
- Represents one of the two most abundant fungal species on human skin, alongside M. globosa
- Constitutes up to 90% of the fungal population in sebaceous skin sites
- Present on virtually all humans regardless of age, gender, or ethnicity
- Particularly abundant on the scalp, face, upper chest, and back
- Also found in lower abundance on arms, legs, and other less sebaceous areas
- Population density increases after puberty when sebum production rises
- Remains a stable component of the skin microbiome throughout adult life
- Shows some seasonal variation, with higher abundance in warmer, more humid conditions
Ecological Niche:
- Primarily occupies the stratum corneum (outermost layer of the epidermis)
- Concentrates around hair follicles and sebaceous gland openings
- Forms part of the complex microbial biofilm on skin surfaces
- Occupies a specialized lipid-dependent niche that few other microorganisms can exploit
- Acts as a primary consumer of sebaceous lipids in the skin ecosystem
- Colonizes humans from early infancy, with populations stabilizing in childhood
- Maintains relatively consistent populations despite daily hygiene practices
- Demonstrates remarkable resilience and ability to recolonize after perturbations
Interactions with Host Skin:
- Metabolizes sebum components, particularly triglycerides and other lipids
- Releases free fatty acids through lipase activity, contributing to skin acidification
- Produces bioactive compounds that can influence skin barrier function
- Interacts with keratinocytes through both direct contact and secreted factors
- Modulates local immune responses through various immunoactive compounds
- May contribute to skin barrier maintenance through lipid processing
- Competes with transient pathogenic microorganisms for space and resources
- Influences sebum composition through selective metabolism of specific lipid components
Interactions with Other Microbiota:
- Engages in complex relationships with bacterial members of the skin microbiome
- Particularly interacts with Cutibacterium acnes (formerly Propionibacterium acnes) in sebaceous sites
- May compete with C. acnes for sebaceous lipids while also potentially benefiting from its lipase activity
- Forms mixed-species biofilms with various skin bacteria
- Produces compounds that can inhibit the growth of certain skin bacteria
- Population dynamics influenced by bacterial community composition
- Metabolic byproducts may serve as nutrients for certain bacterial species
- Ecological balance with other microbiota helps maintain skin homeostasis
Temporal Dynamics:
- Population increases significantly during puberty when sebum production rises
- Remains relatively stable throughout adulthood with minor fluctuations
- May decrease in older adults as sebum production declines
- Shows resilience to short-term perturbations such as washing
- Populations can shift in response to seasonal changes in temperature and humidity
- Demonstrates rapid recolonization following disruption
- Long-term stability suggests well-adapted commensalism with human hosts
- Life stage transitions (infancy, puberty, aging) influence population density
Metabolic Contributions:
- Processes sebaceous lipids, potentially making them more accessible to other microorganisms
- Produces oxylipins and other bioactive lipid mediators that may influence skin physiology
- Contributes to the skin's acid mantle through fatty acid production
- Metabolizes squalene and other sebum components that might otherwise accumulate
- Generates secondary metabolites with potential antimicrobial properties
- May participate in nitrogen cycling on the skin surface
- Contributes to the overall metabolic network of the skin microbiome
- Produces indole compounds with potential immunomodulatory effects
Host-Specific Adaptations:
- Shows evidence of co-evolution with human hosts
- Strain diversity reflects adaptation to different host microenvironments
- Genomic adaptations specifically tailored to human sebum composition
- Optimized for growth at human skin temperature and pH
- Possesses mechanisms to evade or modulate human immune responses
- Adapted to persist despite regular skin shedding and hygiene practices
- Strain-level differences may reflect adaptation to host genetic factors
- Demonstrates remarkable host fidelity compared to environmental fungi
Role in Skin Homeostasis:
- May contribute to maintaining the skin's acidic pH through fatty acid production
- Potentially helps regulate sebum composition through selective lipid metabolism
- Competes with transient pathogenic microorganisms, potentially providing colonization resistance
- Produces compounds that may modulate local immune responses
- Participates in the complex microbial network that maintains skin equilibrium
- May influence keratinocyte turnover and differentiation through secreted factors
- Contributes to the skin's lipid profile through metabolic activities
- Balance between beneficial and potentially harmful effects contributes to skin homeostasis
The role of M. restricta in the human microbiome represents a complex ecological relationship that has evolved over millennia. As a dominant member of the skin mycobiome, it has adapted to thrive in the unique environment provided by human skin, developing specialized mechanisms to acquire nutrients, evade host defenses, and persist throughout the human lifespan. While primarily existing as a commensal, the fine balance between commensalism and pathogenicity can shift under certain conditions, highlighting the dynamic nature of this host-microbe relationship. Understanding these complex interactions is crucial for developing approaches to maintain healthy skin and address Malassezia-associated disorders.
Health Implications
Malassezia restricta has significant implications for human health, ranging from its role as a normal commensal to its involvement in various skin disorders:
Commensal Relationship:
- Present on healthy skin without causing disease in most individuals
- Part of the normal skin microbiome from early infancy throughout life
- May contribute to skin homeostasis through interactions with other microbiota
- Potentially provides colonization resistance against transient pathogens
- Helps maintain the skin's acidic pH through fatty acid production
- Processes sebum components that might otherwise accumulate
- Exists in balance with host immune surveillance in healthy individuals
- Commensal relationship represents evolutionary adaptation between fungus and host
Dandruff and Seborrheic Dermatitis:
- Strongly implicated in the pathogenesis of dandruff and seborrheic dermatitis
- Increased M. restricta abundance often observed in affected individuals
- Metabolizes sebum to produce free fatty acids and other potentially irritating compounds
- Lipase activity releases oleic acid, which can penetrate the skin and disrupt barrier function
- Triggers inflammatory responses in susceptible individuals
- Contributes to the characteristic flaking and inflammation seen in these conditions
- Severity often correlates with fungal load on the scalp
- Antifungal treatments targeting Malassezia are effective in managing these conditions
- Affects approximately 50% of adults worldwide to varying degrees
Atopic Dermatitis:
- Increasingly recognized as a contributor to atopic dermatitis (eczema)
- May act as an allergen in sensitized individuals
- Produces various allergens that can trigger IgE-mediated hypersensitivity
- Contributes to skin barrier dysfunction through lipase activity
- Exacerbates inflammation in already compromised skin
- May influence the course of disease through interactions with the host immune system
- Often found in increased numbers in lesional skin of atopic dermatitis patients
- Contributes to the complex pathophysiology of this multifactorial condition
- Antifungal treatments sometimes provide benefit in Malassezia-sensitized patients
Psoriasis:
- Associated with psoriatic lesions, particularly on the scalp
- May act as an exacerbating factor in genetically predisposed individuals
- Produces compounds that can trigger or amplify inflammatory responses
- Interacts with the dysregulated immune system characteristic of psoriasis
- May contribute to the Koebner phenomenon (development of lesions at sites of trauma)
- Often found in increased numbers in psoriatic plaques
- Relationship is complex and likely involves genetic, immunological, and environmental factors
- Some patients show improvement with antifungal therapy, suggesting a contributory role
Other Dermatological Conditions:
- Implicated in pityriasis versicolor, though M. globosa is more commonly associated
- May contribute to certain forms of folliculitis
- Potentially involved in some cases of rosacea, particularly the papulopustular variant
- Associated with seborrheic blepharitis (inflammation of eyelid margins)
- May exacerbate perioral dermatitis in susceptible individuals
- Potentially contributes to some forms of acne through interactions with C. acnes
- Implicated in otitis externa (inflammation of the external ear canal)
- May play a role in certain nail disorders, particularly in immunocompromised hosts
Systemic and Invasive Infections:
- Rarely causes systemic infections, unlike some other Malassezia species
- Occasionally isolated from bloodstream infections, particularly in premature neonates
- Risk factors for invasive infection include immunosuppression and lipid-rich parenteral nutrition
- Can colonize indwelling medical devices, particularly central venous catheters
- Fungemia typically requires catheter removal and antifungal therapy
- Generally less virulent in systemic infections compared to other fungal pathogens
- Diagnosis challenging due to specialized growth requirements
- Prognosis generally favorable with appropriate treatment and underlying condition management
Immunological Interactions:
- Recognized by the host immune system through pattern recognition receptors
- Cell wall components interact with Toll-like receptors and C-type lectin receptors
- Induces both innate and adaptive immune responses
- Can trigger production of pro-inflammatory cytokines by keratinocytes and immune cells
- Stimulates production of antimicrobial peptides by skin cells
- May induce Th1, Th2, and Th17 responses depending on context
- Produces immunomodulatory compounds that can influence local immune responses
- Balance between immune tolerance and response crucial for maintaining commensalism
Therapeutic Considerations:
- Target of many antidandruff and antiseborrheic treatments
- Susceptible to azole antifungals (ketoconazole, fluconazole) and ciclopirox olamine
- Zinc pyrithione effective through multiple mechanisms including antifungal activity
- Selenium sulfide reduces fungal burden through cytostatic effects
- Coal tar preparations may reduce Malassezia populations and modulate inflammation
- Topical corticosteroids address inflammatory component but not fungal burden
- Emerging approaches target specific virulence factors rather than growth
- Probiotics and microbiome-based therapies being explored as alternative approaches
- Maintenance therapy often required to prevent recurrence of associated conditions
The health implications of M. restricta highlight the complex relationship between this fungus and its human host. While it exists as a harmless commensal in most individuals, various host and environmental factors can tip the balance toward pathogenicity, leading to a spectrum of skin disorders. Understanding the factors that govern this balance is crucial for developing effective preventive and therapeutic strategies. The prevalence of Malassezia-associated conditions, particularly dandruff and seborrheic dermatitis, makes M. restricta a significant organism from a public health perspective, affecting quality of life for millions of people worldwide. Continued research into the biology of M. restricta and its interactions with the host will likely yield new insights and approaches for managing these common conditions.
Metabolic Activities
Malassezia restricta exhibits specialized metabolic activities that reflect its adaptation to the unique ecological niche of human skin:
Lipid Dependency and Acquisition:
- Obligate lipid auxotroph due to inability to synthesize fatty acids de novo
- Lacks genes encoding fatty acid synthase complex, a key evolutionary adaptation
- Requires exogenous sources of long-chain fatty acids (C14 or longer) for membrane synthesis
- Possesses multiple lipid transport systems for efficient uptake from the environment
- Expresses numerous lipases and phospholipases to harvest lipids from sebum
- Preferentially utilizes specific fatty acids, particularly oleic acid (C18:1)
- Capable of incorporating host-derived fatty acids directly into cellular structures
- Lipid acquisition systems are constitutively expressed, reflecting their essential nature
Lipid Metabolism:
- Efficiently metabolizes sebaceous triglycerides, releasing free fatty acids
- Processes complex lipids including wax esters and cholesterol esters
- Metabolizes squalene, a major component of human sebum
- Capable of β-oxidation of fatty acids for energy production
- Produces various lipid-derived metabolites with bioactive properties
- Generates oxylipins through enzymatic modification of polyunsaturated fatty acids
- Lipid metabolism varies between strains, potentially influencing pathogenicity
- Adapts lipid processing based on available substrates in different skin microenvironments
Carbohydrate Metabolism:
- Limited carbohydrate utilization capabilities compared to other fungi
- Preferentially uses lipids over carbohydrates as carbon and energy sources
- Retains core glycolytic pathway despite evolutionary genome reduction
- Possesses truncated tricarboxylic acid (TCA) cycle
- Limited capacity for complex carbohydrate breakdown
- Capable of utilizing glucose and some other simple sugars when available
- Carbohydrate metabolism primarily serves biosynthetic rather than energy needs
- Metabolic flexibility allows adaptation to varying nutrient availability on skin
Nitrogen Metabolism:
- Utilizes various nitrogen sources including amino acids and small peptides
- Expresses proteases and peptidases to harvest nitrogen from host proteins
- Capable of using urea as a nitrogen source, relevant in sweat-rich environments
- Possesses efficient nitrogen scavenging systems adapted to the nitrogen-limited skin environment
- Amino acid biosynthetic pathways largely intact despite genome reduction
- Nitrogen metabolism linked to cell wall synthesis and remodeling
- Efficient recycling of internal nitrogen compounds during nutrient limitation
- Adapts nitrogen acquisition strategies based on microenvironmental conditions
Secondary Metabolite Production:
- Produces various indole derivatives including malassezin, indolo[3,2-b]carbazole, and pityriacitrin
- Generates azelaic acid, which has antimicrobial and anti-inflammatory properties
- Synthesizes various lipid-derived mediators that can modulate host responses
- Produces squalene peroxides through oxidative metabolism of sebum squalene
- Generates volatile organic compounds (VOCs) that contribute to body odor
- Secondary metabolite production varies between strains and growth conditions
- Many metabolites have immunomodulatory properties, influencing host-microbe interactions
- Some metabolites may provide competitive advantages against other microorganisms
Stress Response Metabolism:
- Possesses robust oxidative stress response systems including catalases and superoxide dismutases
- Produces various stress protectants including trehalose and mannitol
- Capable of adapting to the varying temperature, pH, and humidity conditions on skin
- Metabolic adaptations allow survival in the antimicrobial peptide-rich environment of skin
- Stress response pathways integrated with core metabolic networks
- Upregulates protective mechanisms during host inflammatory responses
- Metabolic flexibility contributes to persistence despite host defense mechanisms
- Stress response metabolism particularly important during pathogenic transitions
Biofilm Metabolism:
- Forms biofilms with distinct metabolic profiles compared to planktonic growth
- Produces extracellular matrix components through specialized metabolic pathways
- Exhibits metabolic heterogeneity within biofilm structures
- Adapts to oxygen and nutrient gradients within biofilms
- Biofilm cells show altered lipid metabolism compared to free-living cells
- Metabolic dormancy in biofilm contributes to stress resistance
- Intercellular metabolic cooperation occurs within biofilm communities
- Biofilm metabolism contributes to antifungal resistance and persistence
Interactions with Host Metabolism:
- Metabolizes host-derived lipids, potentially altering skin lipid composition
- Produces metabolites that can influence host cell behavior and immune responses
- Generates free fatty acids that contribute to the skin's acid mantle
- Metabolizes sebum components that might otherwise accumulate and cause irritation
- Produces oxylipins that can modulate inflammatory pathways in host cells
- Metabolic activities influence the growth of other members of the skin microbiome
- Responds to host metabolic signals such as hormones that affect sebum production
- Metabolic adaptation to the host environment reflects long-term co-evolution
The metabolic activities of M. restricta are highly specialized for its ecological niche on human skin. Its obligate lipid dependency represents a key evolutionary adaptation that allows it to exploit the lipid-rich environment provided by sebaceous secretions. This specialization has come at the cost of metabolic versatility, with significant genome reduction and loss of pathways unnecessary for its commensal lifestyle. The metabolic capabilities that remain are finely tuned for efficient acquisition and utilization of available nutrients, stress resistance, and interaction with the host. These metabolic activities not only enable M. restricta to thrive as a dominant member of the skin microbiome but also underlie its potential to transition from commensal to pathogen under certain conditions. Understanding these metabolic processes provides insights into both the basic biology of this important skin commensal and potential targets for therapeutic intervention in Malassezia-associated disorders.
Clinical Relevance
Malassezia restricta has significant clinical relevance across multiple dermatological conditions and occasionally in systemic infections:
Dandruff and Seborrheic Dermatitis:
- Primary fungal species implicated in dandruff and seborrheic dermatitis pathogenesis
- Increased M. restricta abundance correlates with disease severity
- Metabolizes sebum to produce oleic acid and other irritants that disrupt skin barrier
- Triggers inflammatory responses in susceptible individuals
- Antifungal treatments targeting Malassezia are first-line therapy
- Ketoconazole, zinc pyrithione, and selenium sulfide effectively reduce fungal burden
- Affects approximately 50% of adults worldwide to varying degrees
- Often requires maintenance therapy to prevent recurrence
- Disease severity influenced by host factors, fungal load, and strain virulence
- Represents one of the most common fungal-associated human diseases
Atopic Dermatitis:
- Increasingly recognized as a contributor to atopic dermatitis in a subset of patients
- Acts as an allergen in sensitized individuals, triggering IgE-mediated responses
- Specific Malassezia allergens identified and characterized
- Contributes to skin barrier dysfunction through lipase activity
- May exacerbate the characteristic Th2-dominant inflammation
- Head and neck distribution of atopic dermatitis often associated with Malassezia sensitization
- Antifungal therapy sometimes beneficial in Malassezia-sensitized patients
- Diagnostic testing for Malassezia-specific IgE can identify sensitized individuals
- Contributes to the complex pathophysiology of this multifactorial condition
- Represents an important consideration in treatment-resistant cases
Psoriasis:
- Associated with scalp psoriasis and potentially other forms
- May act as an exacerbating factor in genetically predisposed individuals
- Produces compounds that can trigger or amplify inflammatory responses
- Interacts with the dysregulated immune system characteristic of psoriasis
- Potentially triggers Koebner phenomenon in susceptible individuals
- Some patients show improvement with antifungal therapy
- Relationship is complex and likely involves genetic, immunological, and environmental factors
- May influence the Th17-dominant inflammation characteristic of psoriasis
- Represents a potential therapeutic target in certain cases
- Contributes to treatment challenges in scalp psoriasis
Other Dermatological Conditions:
- Implicated in some cases of folliculitis, particularly on the trunk and scalp
- Associated with seborrheic blepharitis (inflammation of eyelid margins)
- May contribute to certain presentations of rosacea
- Potentially exacerbates perioral dermatitis in susceptible individuals
- Involved in otitis externa (inflammation of the external ear canal)
- May play a role in certain nail disorders, particularly in immunocompromised hosts
- Contributes to the complex pathophysiology of acne through interactions with C. acnes
- Implicated in pityriasis versicolor, though M. globosa is more commonly associated
- Represents a consideration in various recalcitrant inflammatory skin conditions
- Often requires specialized diagnostic approaches for confirmation of involvement
Systemic and Invasive Infections:
- Rarely causes systemic infections compared to other Malassezia species
- Occasionally isolated from bloodstream infections, particularly in specific risk groups
- Risk factors include immunosuppression, prematurity, and lipid-rich parenteral nutrition
- Can colonize indwelling medical devices, particularly central venous catheters
- Diagnosis challenging due to specialized growth requirements
- Often requires lipid-supplemented culture media for laboratory isolation
- Treatment typically involves catheter removal and systemic antifungal therapy
- Amphotericin B and azoles are primary treatment options
- Prognosis generally favorable with appropriate treatment
- Represents an important consideration in specific clinical scenarios
Diagnostic Considerations:
- Difficult to culture using standard mycological techniques
- Requires specialized lipid-supplemented media (e.g., modified Dixon's agar)
- Often identified through molecular methods rather than culture
- Metagenomic approaches increasingly used for identification and quantification
- Skin surface sampling techniques include swabbing, scraping, and tape stripping
- Quantitative PCR allows assessment of fungal burden
- Confocal microscopy and other imaging techniques can visualize in situ
- Malassezia-specific IgE testing available for assessing sensitization
- Histopathological examination may reveal yeasts in skin biopsies
- Diagnostic challenges contribute to potential underrecognition of clinical significance
Therapeutic Approaches:
- Topical antifungals effective for most Malassezia-associated conditions
- Ketoconazole available in various formulations (shampoo, cream, foam)
- Ciclopirox olamine provides both antifungal and anti-inflammatory effects
- Zinc pyrithione effective through multiple mechanisms
- Selenium sulfide reduces fungal burden through cytostatic effects
- Systemic antifungals (fluconazole, itraconazole) reserved for severe or recalcitrant cases
- Combination therapy with anti-inflammatory agents often beneficial
- Maintenance therapy frequently required to prevent recurrence
- Emerging approaches target specific virulence factors
- Treatment resistance occasionally observed, requiring alternative strategies
Public Health Impact:
- Malassezia-associated conditions affect billions worldwide
- Significant economic impact through healthcare costs and over-the-counter treatments
- Considerable effect on quality of life, particularly in severe cases
- Psychosocial impact of visible conditions like dandruff and seborrheic dermatitis
- Workplace and social implications of visible skin conditions
- Substantial market for antidandruff and antiseborrheic products
- Growing recognition of importance in common dermatological conditions
- Increasing research interest due to widespread prevalence
- Represents one of the most common human-fungal interactions
- Public education important for appropriate management and treatment expectations
The clinical relevance of M. restricta spans from extremely common conditions like dandruff to rare but serious systemic infections. Its role in common dermatological disorders makes it a significant organism from both medical and public health perspectives. The challenges in culturing and studying M. restricta have historically limited our understanding of its clinical significance, but advances in molecular and genomic techniques are providing new insights. As our understanding of the complex interactions between M. restricta, the host, and other microbiota improves, new diagnostic and therapeutic approaches are likely to emerge. The widespread prevalence of Malassezia-associated conditions ensures that M. restricta will remain clinically relevant for the foreseeable future, with ongoing research efforts aimed at better understanding and managing its role in human health and disease.
Interactions with Other Microorganisms
Malassezia restricta engages in complex interactions with other members of the skin microbiome, influencing community dynamics and potentially affecting skin health:
Interactions with Cutibacterium acnes:
- Co-exists with C. acnes (formerly Propionibacterium acnes) in sebaceous follicles
- Potential competition for sebaceous lipids as both organisms are lipophilic
- May benefit from C. acnes lipase activity, which releases free fatty acids from triglycerides
- C. acnes produces propionic acid that may influence M. restricta growth
- Both organisms contribute to the processing of sebum components
- Ecological balance between these species may influence follicular health
- Disruption of this balance potentially contributes to conditions like acne and seborrheic dermatitis
- Metabolic byproducts from each organism may influence the other's growth and virulence
- Co-aggregation observed in biofilm communities
- Relative abundance shifts with age, sebum production, and in disease states
Interactions with Staphylococcus Species:
- Co-exists with various Staphylococcus species, particularly S. epidermidis
- S. epidermidis produces antimicrobial peptides that may modulate M. restricta growth
- M. restricta may influence staphylococcal biofilm formation through secreted factors
- Competition for space and nutrients on skin surface
- Potential synergistic interactions in certain disease states
- S. epidermidis may provide protection against more pathogenic microbes
- Both contribute to the skin's antimicrobial defense through different mechanisms
- Ecological balance important for maintaining healthy skin
- Disruption of this balance may contribute to inflammatory skin conditions
- Relative abundance varies by body site and microenvironment
Interactions with Other Malassezia Species:
- Co-exists with other Malassezia species, particularly M. globosa
- Potential competition for similar ecological niches and resources
- Different species show preferences for specific lipid profiles
- M. restricta generally dominates in drier areas, while M. globosa prefers more sebaceous sites
- Species distribution varies by body site, age, and geographic region
- Potential metabolic cooperation or competition between species
- Relative abundance shifts may be associated with various skin conditions
- Different species may exhibit complementary roles in skin ecology
- Interspecies interactions potentially influence virulence expression
- Genetic exchange between species theoretically possible but not well documented
Interactions with Corynebacterium Species:
- Co-exists with various Corynebacterium species in moist skin areas
- Corynebacteria metabolize sweat components, potentially altering the microenvironment
- May influence each other's growth through secreted factors
- Both contribute to body odor through different metabolic pathways
- Potential competition for certain micronutrients
- Co-aggregation in multispecies biofilms
- Ecological balance varies by body site and environmental conditions
- Disruption of balance may contribute to conditions like erythrasma
- Metabolic complementarity may exist in certain microenvironments
- Relative abundance influenced by hygiene practices and environmental factors
Biofilm Communities:
- Forms part of polymicrobial biofilms on skin surfaces
- Contributes to biofilm structure and function through secreted factors
- Biofilm formation enhances resistance to environmental stressors
- Spatial organization within biofilms influenced by species interactions
- Metabolic cooperation and nutrient exchange within biofilm communities
- Quorum sensing may coordinate behaviors across species
- Biofilms provide protection against antimicrobial agents and host defenses
- Mixed-species biofilms show enhanced stability compared to single-species biofilms
- Biofilm lifestyle predominates in many skin microenvironments
- Disruption of healthy biofilm communities may contribute to dysbiosis and disease
Competition for Resources:
- Competes with other microorganisms for limited nutrients on skin
- Particularly competes for lipids with other lipophilic organisms
- Competition for adhesion sites on corneocytes
- Competition for micronutrients such as iron, zinc, and copper
- Produces compounds that may inhibit competitors
- Specialized adaptations for efficient nutrient acquisition
- Resource partitioning may allow coexistence of multiple species
- Competition intensity varies with resource availability
- Competitive dynamics influenced by host factors and environmental conditions
- Outcome of competition may influence community structure and function
Metabolic Interactions:
- Cross-feeding relationships where metabolic byproducts of one organism serve as nutrients for another
- M. restricta may utilize fatty acids released by bacterial lipases
- Produces metabolites that can influence the growth and behavior of other microorganisms
- Modifies the microenvironment through pH changes and secreted factors
- Oxygen consumption may create microaerobic niches for other organisms
- Metabolizes sebum components, potentially making them more accessible to other microbes
- Detoxifies certain compounds that might inhibit other community members
- Metabolic complementarity contributes to community stability
- Syntrophic relationships may exist with specific bacterial species
- Metabolic interactions contribute to the overall functional capacity of the skin microbiome
Influence on Host-Microbe Interactions:
- Presence may modulate how other microorganisms interact with the host
- Immunomodulatory compounds produced by M. restricta can affect host responses to other microbes
- May influence skin barrier function, affecting colonization by other organisms
- Interactions with other microbes can amplify or suppress inflammatory responses
- Contributes to colonization resistance against transient pathogens
- Polymicrobial interactions may influence transition from commensalism to pathogenicity
- Host genetic factors influence microbial interactions and community structure
- Environmental factors such as hygiene practices affect interspecies dynamics
- Therapeutic interventions targeting one community member may have cascading effects
- Understanding these complex interactions crucial for microbiome-based therapeutic approaches
The interactions between M. restricta and other microorganisms represent a complex ecological network that influences both microbial community dynamics and host health. These interactions range from competition for resources to metabolic cooperation, collectively contributing to the stability and function of the skin microbiome. Disruption of these balanced interactions, through factors such as antibiotic use, changes in sebum production, or altered immune function, may contribute to dysbiosis and associated skin conditions. As research continues to elucidate the intricate relationships within the skin microbiome, new opportunities for therapeutic approaches targeting microbial interactions rather than individual species may emerge. The ecological perspective on M. restricta's role in the skin microbiome highlights the importance of considering the entire microbial community when studying skin health and disease.
Research Significance
Malassezia restricta holds substantial research significance across multiple scientific disciplines, with implications for basic biology, dermatology, microbiology, and therapeutic development:
Evolutionary and Genomic Insights:
- Model organism for studying fungal adaptation to the human host
- Exemplifies extreme genomic reduction and metabolic specialization
- Provides insights into evolution of obligate host-associated fungi
- Comparative genomics with other Malassezia species reveals adaptive strategies
- Study of lipid dependency illuminates essential fungal metabolic pathways
- Genome analysis reveals mechanisms of adaptation to the skin environment
- Horizontal gene transfer events suggest complex evolutionary history
- Strain diversity studies provide insights into population structure and dynamics
- Represents a unique evolutionary trajectory among pathogenic fungi
- Genomic insights may inform understanding of other host-adapted microorganisms
Skin Microbiome Research:
- Dominant fungal component of the human skin microbiome
- Model for studying fungal-bacterial interactions in a natural ecosystem
- Contributes to understanding temporal and spatial dynamics of skin microbial communities
- Helps define the concept of "healthy" versus "dysbiotic" skin microbiome
- Provides insights into host factors that shape microbiome composition
- Model for studying biofilm formation and function in a natural setting
- Contributes to understanding of microbiome development throughout life stages
- Illuminates host-microbe-environment interactions on skin
- Advances metagenomic approaches for studying difficult-to-culture organisms
- Contributes to the broader field of human microbiome research
Host-Microbe Interactions:
- Model for studying immune tolerance to commensal fungi
- Illuminates mechanisms of transition from commensalism to pathogenicity
- Provides insights into fungal recognition by the innate immune system
- Helps define the role of pattern recognition receptors in fungal sensing
- Model for studying host-microbe communication through metabolites
- Advances understanding of skin barrier function in microbial defense
- Contributes to knowledge of microbial influence on skin immune homeostasis
- Illuminates mechanisms of IgE-mediated hypersensitivity to commensal fungi
- Model for studying trained immunity in the skin
- Advances understanding of host-microbe co-evolution
Dermatological Disease Mechanisms:
- Critical for understanding pathogenesis of dandruff and seborrheic dermatitis
- Contributes to knowledge of inflammatory mechanisms in various skin disorders
- Helps define the role of fungi in atopic dermatitis
- Provides insights into microbial contributions to psoriasis
- Advances understanding of sebaceous gland function and dysfunction
- Illuminates mechanisms of skin barrier disruption in inflammatory conditions
- Contributes to knowledge of microbial factors in chronic skin inflammation
- Helps define the complex interplay between host genetics and microbial factors
- Advances understanding of recalcitrant dermatological conditions
- Provides insights into age-related changes in skin health and disease
Therapeutic Development:
- Target for antifungal and antiseborrheic drug development
- Model for developing microbiome-based therapeutic approaches
- Contributes to understanding of antifungal resistance mechanisms
- Helps identify novel drug targets for Malassezia-associated conditions
- Advances development of immunomodulatory approaches for skin disorders
- Contributes to formulation science for topical antifungal delivery
- Provides insights for developing targeted antimicrobial peptides
- Model for testing prebiotic and probiotic approaches for skin health
- Advances understanding of maintenance therapy requirements
- Contributes to development of biomarkers for treatment response
Methodological Advances:
- Drives development of specialized culture techniques for lipid-dependent fungi
- Advances molecular methods for detection and quantification of skin fungi
- Contributes to metagenomic approaches for studying complex microbial communities
- Helps develop imaging techniques for visualizing microbes in skin
- Advances models for studying host-microbe interactions in vitro
- Contributes to development of skin equivalent models incorporating microbiome
- Drives innovation in sampling techniques for skin microbiome research
- Advances methods for functional characterization of uncultivable microbes
- Contributes to development of strain typing and tracking methods
- Helps establish standards for skin microbiome research
Industrial and Commercial Applications:
- Target for personal care product development (antidandruff shampoos, etc.)
- Contributes to development of cosmetic ingredients for skin health
- Advances understanding of microbial contribution to body odor
- Helps develop testing methods for antimicrobial efficacy
- Contributes to formulation of skin care products for specific conditions
- Advances development of diagnostic tools for skin disorders
- Helps establish standards for claiming antimicrobial efficacy
- Contributes to understanding of product effects on skin microbiome
- Advances development of microbiome-friendly personal care products
- Significant economic impact through personal care product market
Future Research Directions:
- Integration of multi-omics approaches to understand M. restricta in its ecological context
- Development of genetic manipulation systems for functional genomics
- Exploration of strain-specific virulence factors and host interactions
- Investigation of potential beneficial roles in skin health
- Development of targeted approaches to modulate rather than eliminate M. restricta
- Exploration of interactions with the skin virome and its implications
- Investigation of potential systemic effects beyond the skin
- Development of personalized approaches based on host and microbial factors
- Exploration of environmental influences on M. restricta populations
- Investigation of potential applications in biotechnology and industrial processes
The research significance of M. restricta extends from fundamental questions about fungal biology and evolution to practical applications in medicine and personal care. As a dominant member of the human skin microbiome with clear associations to common skin disorders, M. restricta represents an important model organism for understanding host-microbe interactions in health and disease. The challenges in studying this fastidious organism have historically limited progress, but advances in genomic, metagenomic, and imaging technologies are rapidly expanding our knowledge. Continued research on M. restricta promises to yield insights that will advance our understanding of the skin ecosystem and lead to improved approaches for maintaining skin health and treating Malassezia-associated disorders. The widespread prevalence of these conditions ensures that M. restricta will remain a significant focus of research attention for the foreseeable future.
